A research group led by Associate Professor Hiroki Miyaoka of Hiroshima University Natural Science Center for Basic Research and Development and Professor Takayuki Ichikawa of Hiroshima University Graduate School of Advanced Science and Engineering announced the development of an efficient technique to control the process of ammonia synthesis at atmospheric pressure using LiH. The high nitrogen dissociation ability of Li was the focus of this work. Ammonia synthesis was successfully achieved at ambient pressure via a multi-step chemical reaction process that is different from the conventional noble metal catalytic process. Since it can be synthesized in small-scale facilities, it is expected to be used as a new option for hydrogen storage facilitating the utilization of hydrogen energy for the decarbonization of society. The results were published in the international scientific journal "The Journal of Physical Chemistry C".
(Image courtesy of Hiroki Miyaoka, Hiroshima University)
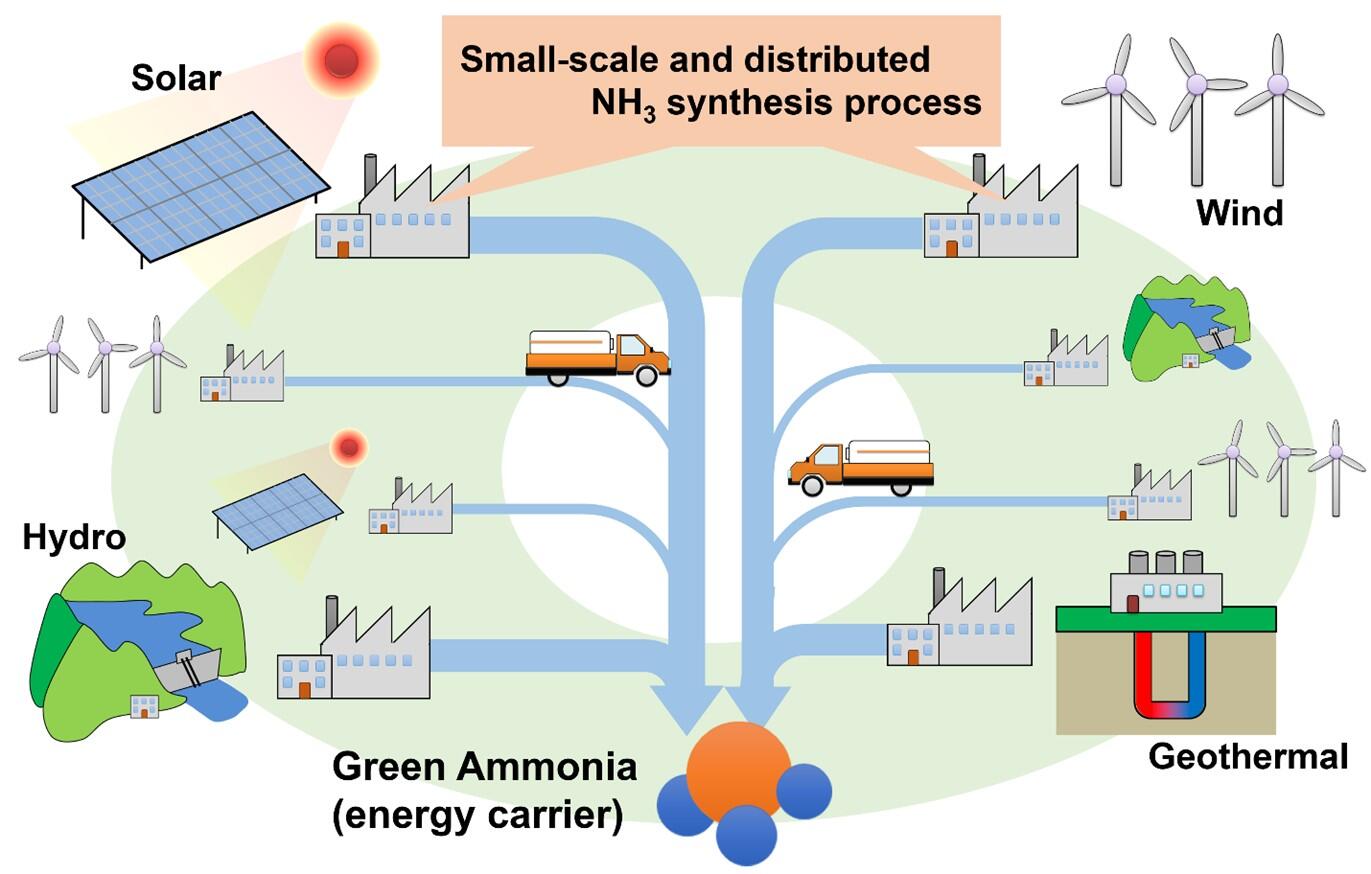
In October 2020, the government announced that it would work to make Japan carbon neutral and entirely eliminate greenhouse gas emissions by 2050. In the 6th edition of Japan's basic energy plan published last April, in view of the carbon neutral era, it was stated that hydrogen would be positioned as a new resource, and that the societal implementation of ammonia utilization as a storage and transport medium would be advanced. Currently, the Haber-Bosch process, which is performed under high-temperature and high-pressure conditions of approximately 500 °C and 250 atm or higher, is used for the synthesis of ammonia (NH3); however, this method is intended for large-scale production of fertilizers, and other products. The storage and transport of excess hydrogen is costly, and therefore, assuming that hydrogen production is performed using ubiquitous natural renewable energy sources, techniques for distributed small-scale NH3 synthesis that can be controlled under lower temperature and pressure conditions are required. So far, the research group has clarified that Li has a high nitrogen dissociation ability, and has developed and presented an ammonia synthesis method using Li alloy based on reactions that proceed at atmospheric pressure.
Therefore, this study group focused on the high nitrogen dissociation ability of Li. A chemical looping process was developed to synthesize NH3 while recycling LiH via a multistep chemical reaction that is distinct from the conventional process using noble metal catalysts. First, an experiment with the aim of understanding and controlling the NH3 synthesis process using LiH, consisting of the following two steps, was undertaken:
- Reaction (1) dissociation: 4LiH + N2 → 2Li2NH + H2
- Reaction (2) NH3 synthesis/ regeneration: 2Li2NH + 4H2 → 2NH3 + 4LiH
When several millimeters of powdered LiH was used, under 0.1 MPa (atmospheric pressure) conditions, the reaction rate was approximately 60% when heated to 500 °C and reached approximately 80% when the same conditions were maintained for 20 h. To investigate the cause, after reaction (1), the sample was observed using scanning electron microscopy, and it was found that the product was aggregated with unreacted reagent. To suppress the aggregation, the group considered adding a chemically stable substance as a molecular scaffold.
In reaction (1), Li2O was mixed with LiH. As a result, the reaction rate was enhanced by Li2O acting as a molecular scaffold, reaching 100% reaction rate in 20 min. It was also confirmed that the aggregation was suppressed in the sample after the reaction. Mixing LiO with LiH also increased the reaction rate of reaction (2), which reached 100%. In reaction (2) NH3 was produced at 260 °C, and the regeneration of LiH was also confirmed. This technique can be miniaturized because NH3 can be circulated and synthesized by introducing nitrogen and hydrogen gases independently while switching to the reaction vessel. As it is an exothermic reaction, it is considered that self-sustaining control is possible in principle except for the initial heating. In the future, the group will study the reaction control processes including thermal management, evaluate the characteristics in practical applications, and search for cheaper Li2O alternatives. The researchers have already obtained a patent and aim to establish the technology in the next 1-2 years.
According to Associate Professor Miyaoka: "One significant advantage of chemical looping technology is that, unlike existing technology, nitrogen and hydrogen do not need to be placed in the container simultaneously. I believe that the ability to synthesize ammonia while switching the reaction between nitrogen and hydrogen is an advantageous control technology. In the future, we would like to study how to control and manage the heat, which is a problem."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




