Acetylene gas is an important substance used in acetylene burners and as a chemical raw material but requires the use of very heavy cylinders with solvents due to the danger of exploding when compressed above two atmospheric pressures. Moreover, since the gas is mixed with solvents during extraction, it results in low acetylene purity, so refining equipment was necessary to use it as a raw chemical material.
A research group led by Director and Distinguished Professor Susumu Kitagawa and Research Assistant Professor Ken'ichi Otake at the Institute for Integrated Cell-Material Sciences (iCeMS), Kyoto University, in collaboration with Air Liquide, has successfully developed a porous material that enables the safe storage and transport of large amounts of highly pure acetylene.
"In nature, there is an S-shape adsorption mechanism that changes its structure depending on adsorption, and we designed a PCP (porous coordination polymer) with a flexible structure based on this mechanism," says Dr. Otake. "We were also able to demonstrate that a prototype test unit made by Air Liquide can store a large amount of acetylene at 1.5 atmospheres. We would like to develop this for various gases in the future." The research group's findings were published in Nature Chemistry online.
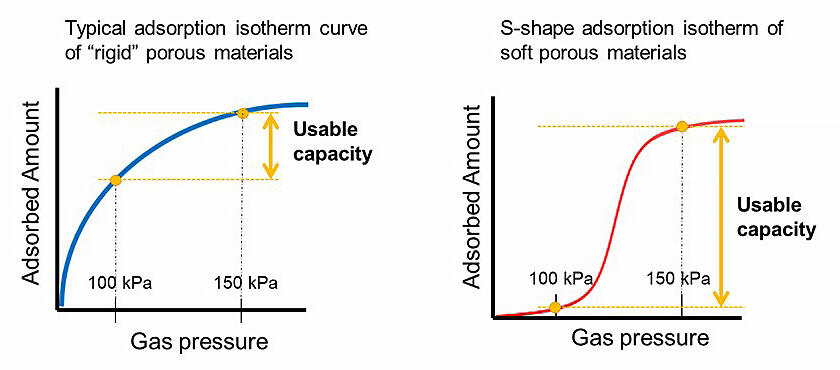
In nature, the mechanism for gas storage and transport is an S-shape adsorption property in which hemoglobin changes its structure as it adsorbs oxygen to efficiently transport gases in large quantities. The adsorption isotherm of typical adsorption materials, such as silica and zeolite, rises parabolically, but PCP with its flexible structure has an S-shape adsorption isotherm that rapidly increases in adsorption at a certain pressure. The research group thought that if they could develop an S-shape adsorption material that shows adsorption/desorption between one and two atmospheres, it would be very promising as a solid packing agent for acetylene cylinders.
Provided by Kyoto University iCeMS
The PCP is a crystalline porous material with a repeating structure consisting of organic molecules and metal ions as parts, with numerous small holes arranged in a regular and orderly manner. Unlike common porous materials such as zeolite and activated carbon, the pore structure can be designed to be functional.
The group synthesized a mixture of PCP, a mix of zinc ion and 4,4-bipyridyl with terephthalic acid and 2-amino terephthalic acid (or 2-nitro terephthalic acid) in arbitrary ratios and systematically investigated its adsorption properties. The PCP has a jungle gym-like network of interpenetrating organic molecules and metal ions and exhibits gate-opening behavior for acetylene. They also discovered that a wide range of adsorption and desorption pressures can be precisely controlled by changing the ratio of functional groups introduced into the skeleton.
In addition, a prototype cylinder made by Air Liquide confirmed that the PCP adsorbs a large amount of acetylene at 1.5 atmospheres and releases it at one atmospheric pressure. The available capacity at 1.5 atmospheres is the largest of any porous material reported to date.
Conventional cylinders can store 1,400 liters of acetylene but require 19 atmospheres of pressure, while the acetylene concentration extracted is less than 95%. The prototype cylinder has a small capacity of 700 liters but can store the gas at 1.5 atmospheres while allowing 99.99% of the acetylene gas to be extracted by simply opening a valve. Because the pressure is low, it does not need to be made of thick steel like conventional cylinders, so it can be made lighter and larger.
"Acetylene is an important substance for chemical synthesis, but there was no way to transport high-purity acetylene," said Director Kitagawa. "Until now, acetylene could not be used as a material for chemical synthesis because it could not be transported in large quantities and its purity was low. If the newly developed cylinder can be used, organic synthesis using acetylene as a chemical raw material, which has not progressed to date, will advance rapidly."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




