A research group led by Director and Project Leader Tetsuya Yamaki, Senior Researcher Shunya Yamamoto, and Senior Researcher Tetsuya Kimata of the Takasaki Advanced Radiation Research Institute, Quantum Beam Science Research Directorate, National Institutes for Quantum Science and Technology, Specially Appointed Researcher Wei Mao and Professor Emeritus Takayuki Terai of the Graduate School of Engineering, the University of Tokyo, Research Director Daiju Matsumura and Research Director Iwao Shimoyama of the Japan Atomic Energy Agency (JAEA), and others has successfully improved the catalytic performance of polymer electrolyte fuel cells (PEFCs) by more than twofold using a new method of retaining platinum on ion beam irradiated carbon materials. The group also shed light on the mechanism responsible for this performance improvement due to the interaction between the defects introduced into the carbon material and platinum (Pt) particles.
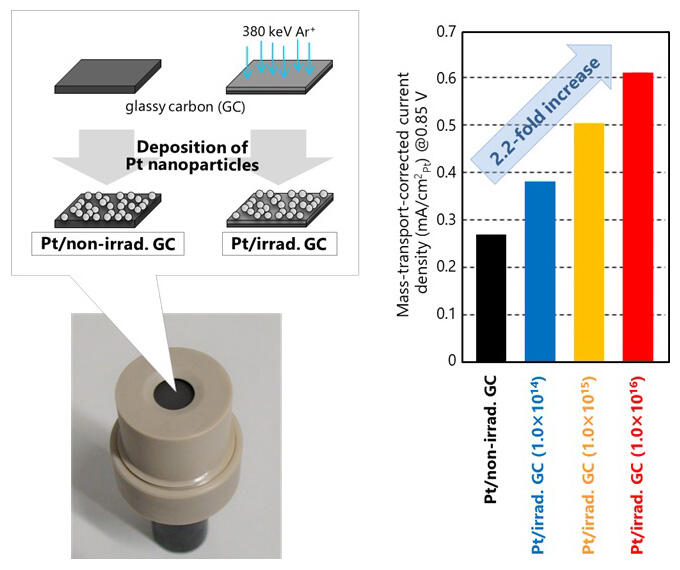
(right) a 2.2-fold increase in oxygen reduction reaction (ORR) activity for Pt/irrad. GC. was found
Provided by QST
Based on previous theoretical predictions, the research group found that the redox reaction (ORR) activity of carbon materials irradiated with ion beams and loaded with Pt particles is enhanced. According to Dr. Yamaki, "We were skeptical at first, but the fact that there was no change in the size or dispersibility of the platinum particles led us to expect that the electronic structure was manipulated by defects introduced into the carbon material." "After examining how to turn this expectation into certainty, we thought that a shortcut would be to use synchrotron radiation experiments to identify the high activation mechanism," he adds.
In their research, the research group attempted to manipulate the electronic structure of platinum particles to produce highly active ORR catalysts by taking advantage of the Pt-carbon interaction enhanced by defects in the lattice structure of carbon materials. The amount of defect structures was controlled by adjusting the number of irradiated argon ions between 1.0 × 1014 and 1016 per square centimeter. The ORR activity of Pt particles (Pt/irradiated GC) deposited on glassy carbon (GC) substrates containing lattice defects was evaluated by the rotating electrode electrochemical technique.
The results showed that in the relationship between current and potential per Pt unit area, which represents activity, the current increases with irradiation of the GC substrate when compared at the same potential. For example, a comparison of the current at a potential of 0.85 V reveals that the activity simply increases with the number of irradiated ions, i.e increasing the number of defects, up to about 2.2 times higher than for Pt particles on unirradiated substrates (Pt/unirradiated GC).
The research group is currently conducting a parallel study on durability, aiming to achieve a 10-fold improvement in ORR activity × durability performance.
"In diamond, which is also made of carbon, defects in the structure such as atomic vacancies have been applied to quantum sensors, so we are also focusing on this achievement as a part of quantum technology," says Dr. Yamaki. "We are hopeful that the localization effect of the defect structure can be used to develop ORR catalysts that do not contain any platinum at all."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




