Hydrogenation reactions of aromatic rings such as benzene have practical hydrogen storage and transport applications. They are also vital for synthesizing functional molecules such as pharmaceuticals and bioactive substances. A research group led by Professor Shu Kobayashi and Assistant Professor Hiroyuki Miyamura at the University of Tokyo's Department of Chemistry, Graduate School of Science, has discovered that a newly developed cooperative catalyst system is approximately 30 times more effective in accelerating these reactions than conventional methods.
Provided by the University of Tokyo with modifications
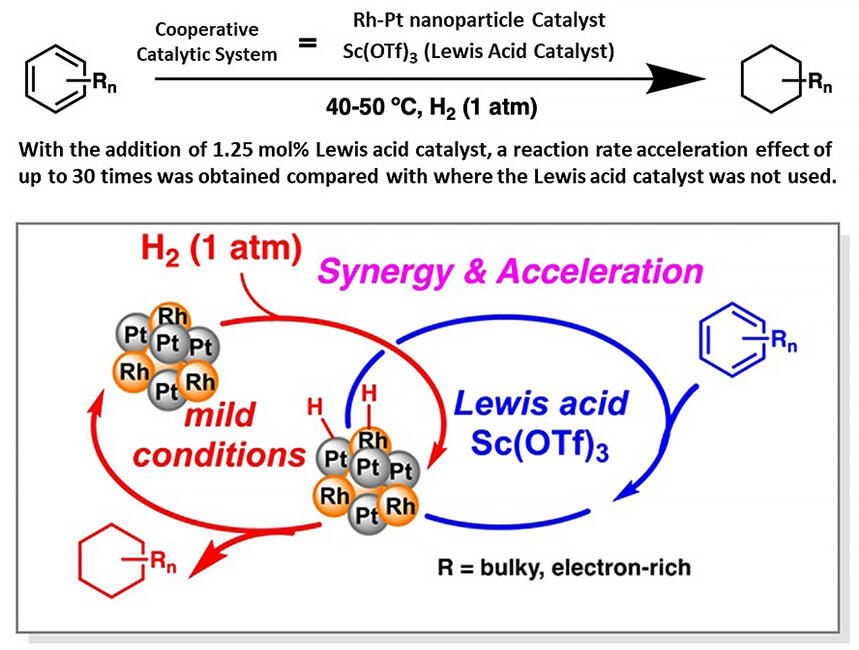
Hydrogenation reactions of aromatic compounds are important for the synthesis of pharmaceuticals and other high value-added chemicals and for hydrogen storage and transportation--which are barriers to the realization of a hydrogen-based society--using the organic chemical hydride method. Hydrogenation of aromatic compounds with multiple bulky or electron-rich substituents is challenging. It requires harsh reaction conditions such as high temperatures and pressures, making the development of an efficient synthetic method a challenge.
The research group developed a heterogeneous rhodium-platinum bimetallic (Rh/Pt) nanoparticle catalyst on an inexpensive organic-inorganic hybrid carrier and a cooperative catalytic system consisting of scandium triflate, a Lewis acid that is stable in water and alcohol-based solvents. These catalysts were applied to the hydrogenation of aromatic rings. This catalytic system works under mild conditions and can be used for aromatic compounds with multiple substituents that are sterically intricate or bulky and electron-rich, types that are particularly difficult to hydrogenate using conventional methods.
The group found that difficult-to-hydrogenate aromatic compounds were smoothly hydrogenated at 1 atmosphere of hydrogen and low temperatures (below 50°C) due to the cooperative effects of the heterogeneous catalysts (Rh-Pt nanoparticles and Lewis acid catalyst). It was also found that this cooperative catalyst system was up to 30 times more effective in accelerating the reaction than conventional methods. These results provide a new method for the highly efficient supply of valuable materials.
Professor Kobayashi said, "In the future, we expect to contribute to the achievement of the SDGs by developing this catalyst system into a continuous flow process, which will bring it closer to practical use and help achieve resource and energy savings in the synthesis of pharmaceuticals and other chemical products. The successful development of this catalytic reaction has also paved the way for developing various aromatic compounds, which have been difficult to hydrogenate, as new hydrogen carriers for hydrogen transport."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




