A research group led by Assistant Professor Yoshihiko Furuike, Assistant Assistant Professor Atsushi Mukaiyama, Professor Shuji Akiyama, and Researcher Dongyan Ouyang of the Institute for Molecular Science, Senior Researcher Tatsuhito Matsuo and Researcher Satoru Fujiwara of the National Institutes for Quantum Science and Technology (QST), Associate Researcher Taiki Tominaga of the Comprehensive Research Organization for Science and Society (CROSS), and Principal Scientist Yukinobu Kawakita at the Japan Atomic Energy Agency (JAEA), has observed the motions of the protein KaiC in Cyanobacteria using neutrons to investigate the mechanism by which the circadian cycle of living organisms is maintained constant regardless of temperature. Through their observations, they succeeded in identifying that clock proteins realize a precise timekeeping system by taking advantage of the characteristics of atomic and whole-molecule motions.
Provided by NINS/IMS
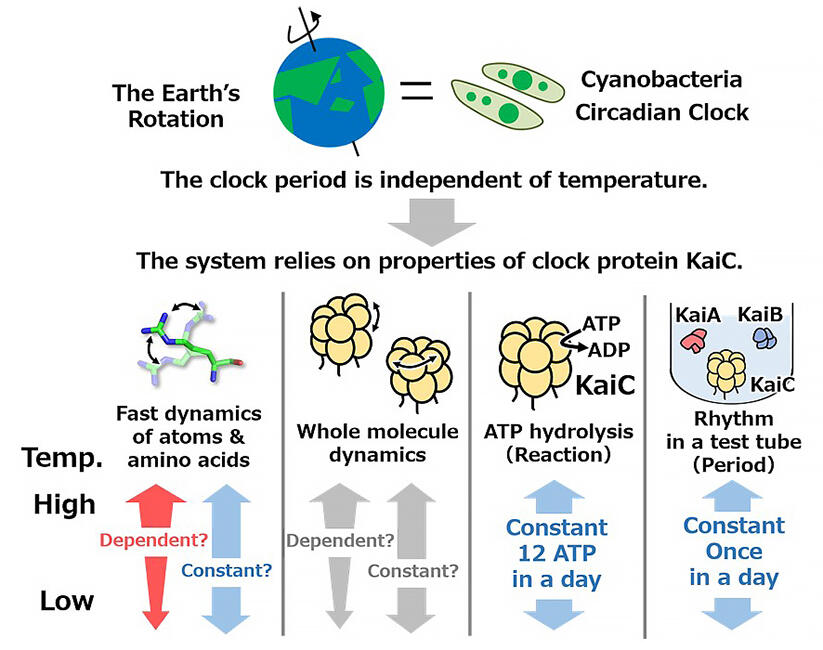
KaiC is an enzyme that plays a central role in the circadian clock system (also known as the biological clock), which marks the rhythm of the 24-hour cycle. KaiC counteracts the effects of temperature changes and maintains a constant enzyme activity (ATP hydrolysis activity, ATPase) in the physiological temperature range, keeping the advancement of the body clock steady. However, little is known about how KaiC maintains constant ATPase activity regardless of temperature.
The research group designed amino acid mutants of KaiC with impaired temperature compensation (KaiC mutant) that were useful for comparison with temperature compensated wild KaiC. These KaiC mutants include those whose rhythms accelerate with increasing temperature (accelerated type) and those whose rhythms conversely slow down with increasing temperature (moderated type). They conducted neutron quasi-elastic scattering experiments on these temperature-sensitive KaiC mutants at the Materials and Life Science Experimental Facility of the Japan Proton Accelerator Research Complex (J-PARC).
From this, it was found that increasing the temperature by 10°C accelerated the motion of the amino acid side chains that make up KaiC by 1 to 2-fold. The results confirmed for KaiC temperature-dependent mutants that lost their temperature compensation ability by substituting amino acid and general proteins other than KaiC. For the whole-molecule motion of KaiC, they observed a significant slowdown only in the KaiC temperature-dependent mutants. These findings indicate that KaiC senses temperature changes and changes its overall molecular motion to compensate for them, as it advances the hands of its circadian clock at a constant rate regardless of day or night temperatures.
The circadian clock, which controls the time in living things, is moderated so that the rate at which the hands move forward (24-hour cycle) is not affected by temperature. The present study shows that clock proteins are not completely unaffected by temperature, but rather, by being affected, they sense temperature changes and autonomously change their overall molecular motions to compensate for them.
"Mechanisms that maintain a constant state regardless of the surrounding environment can be seen in biological homeostasis (body temperature, blood sugar, etc.) in the life sciences and in constant value control (air conditioners and aircraft attitude control) in the field of engineering. It is amazing to know that this is also seen in a molecule as small as 10 nanometers," commented Dr. Akiyama. "If we can expand our observations to clock proteins of other species and confirm that this phenomenon is universal, we may be able to design ultramicro-constant control devices using protein molecules as materials based on these observations. Going into the future we expect to develop techniques that allow us to handle smaller quantity and lower concentration samples, and to improve the research environment to enable the use of neutron beams with higher brilliance."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




