The research group consisting of Senior Lecturer Takahiro Yamashita, Postdoctoral Fellow Kazumi Sakai, Professor Emeritus Yoshinori Shichida, and Associate Professor Yasushi Imamoto of the Department of Biophysics, Graduate School of Science, Kyoto University, has succeeded in developing a new molecular tool that uses light to modify the eye's photoreceptor proteins to transiently change levels of cAMP (cyclic adenosine monophosphate), which plays an important role in many cells.
Provided by Kyoto University
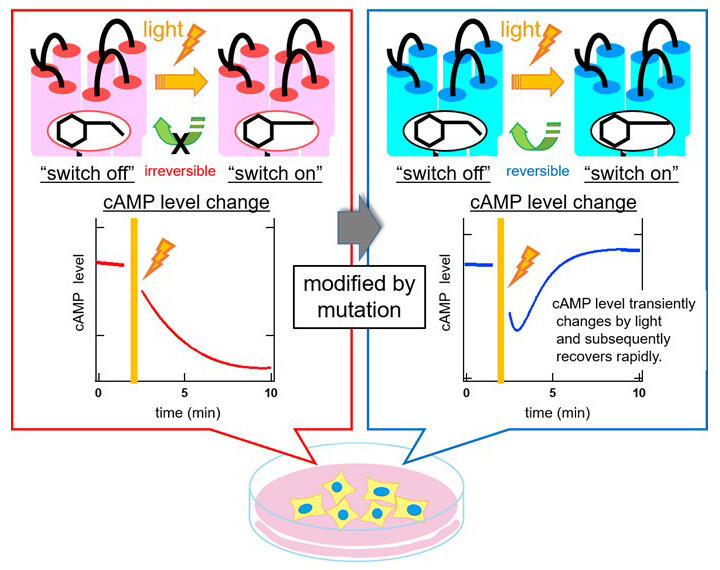
In many animal cells, cAMP is involved in important functions such as differentiation, subsistence, polarity formation, and hormone secretion. Therefore, if cAMP levels can be changed by light without directly touching the cells, then their functions can "remotely controlled" at will. Rhodopsin, a photoreceptive protein that works in the eye, was known to be able to induce changes in cAMP levels via light, but there were several challenges in using it for remote control. The research group attempted to create an amino acid mutation in visual rhodopsin that, similar to channelrhodopsin, switches on with light and then spontaneously switches off. In the process of doing so, the research group previously discovered an opsin (Opn5L1), which differs from visual rhodopsin. This is the only opsin possessed by animals that have photocyclic properties, and the research group had already found the key important amino acid residue that brings about those properties.
Using this information, the group introduced this amino acid residue into visual rhodopsin and found that it became possible to confer photocyclic properties where it spontaneously switches off and returns to its original state after being switched on by light. Furthermore, it was also found that, with the introduction of another mutation, the time before it spontaneously switches off could be changed. When experiments were actually conducted using cells derived from humans, it was confirmed that this modified visual rhodopsin was able to temporarily reduce intracellular cAMP levels with light and that afterwards they then returned to the original levels in a short period of time. The photoreceptive protein that the research group created can be used as a new molecular tool that causes transient changes in cAMP levels for short periods of time.
Senior Lecturer Yamashita said, "Besides cAMP, there are many substances that play important roles in the cell. Going forward, we would like to utilize eye photoreceptor protein in even more ways to change the concentration of various substances and make it easier to remotely control functions in cells other than the eye.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




