A research group led by Assistant Professor Hikaru Fujita and Professor Munetaka Kunishima of the Faculty of Pharmaceutical Sciences, Institute of Medical, Pharmaceutical, and Health Sciences, Kanazawa University, has succeeded for the first time in the world in synthesizing tetraphenylammonium, a "phantom ion" with a very basic chemical structure but whose existence had yet to be confirmed.
Because of its simple and mundane structure, the existence of tetraphenylammonium was rarely questioned, much less fully recognized as challenging to synthesize. However, since it was expected to have properties such as excellent stability and improved reactivity of the paired anions when forming salts, the research group started the process of synthesis to clarify these properties.
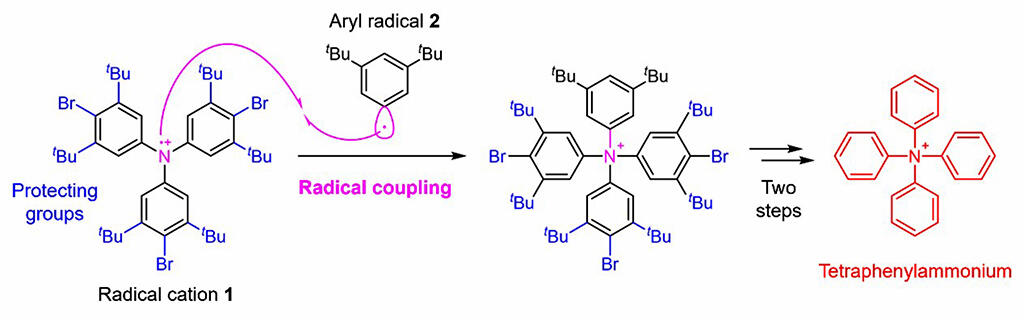
In this study, the research group developed a novel synthesis strategy and succeeded in synthesizing tetraphenylammonium. The key point in the synthesis of this molecule is how to bond the fourth phenyl group to the nitrogen atom with three phenyl groups. Applying a technique called radical coupling, their strategy was for an intermolecular radical coupling reaction between an aryl radical 2 and a triarylammoniumyl radical cation 1. (Fig) This resulted in successfully achieving the desired chemical transformation, albeit in a low yield of 0.1%.
Provided by Kanazawa University
Such radical coupling has the advantage of also enabling the formation of bonds that cannot be achieved by other methods, since bonds are formed between highly reactive radicals. On the other hand, its disadvantage is that the excessively high reactivity makes it difficult to control selectivity, and various side reactions can occur. In the group's synthesis, they devised a method of introducing a protective group that causes steric hindrance in order to suppress the side reaction that results in the formation of a bond on the carbon of the radical cation as much as possible. Finally, they performed chemical transformations, such as introduction of protecting groups, radical coupling and removal of protecting groups, on known triphenylamine derivatives as synthetic raw materials, leading to tetraphenylammonium.
The structure of the synthesized tetraphenylammonium was determined by X-ray crystallography and had a bond distance of only 1.529 Å between the nitrogen atom in the ion and the phenyl group carbon. Since this bond distance is shorter than that of tetraphenyl-type structures containing other elements (boron, carbon, aluminum, silicon, and phosphorus), the tetraphenylammonium nitrogen atoms are in a more spatially crowded environment than those of other elements.
In addition, the results of this study have revealed that tetraphenylammonium is highly stable and can withstand strongly acidic and basic conditions.
"In the future, we would like to develop more efficient and generalized synthesis methods based on the knowledge we have obtained and synthesize a variety of tetraphenylammonium compounds consisting of aromatic rings with various substituents, as well as to learn their physical and chemical properties," says Professor Kunishima. "We would like to consider developing applications in a wide range of fields, including organic chemistry, electrochemistry, surface chemistry, materials chemistry, polymer chemistry, and catalytic chemistry."
■ Radical coupling: A reaction in which two radicals combine. While electrons in a molecule generally form as a pair, radicals have unpaired, highly reactive electrons (unpaired electrons) and are therefore prone to react to form bonds with other radicals to create shared electron pairs. The synthesis reaction in this study used radical cations, which are positively charged radicals.
Journal Information
Publication: Nature Communications
Title: Synthesis and characterization of tetraphenylammonium salts
DOI: 10.1038/s41467-022-30282-y
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




