A research group led by specially appointed professor Katsumi Kaneko of Research Initiative for Supra-Materials (RISM), Interdisciplinary Cluster for Cutting Edge Research, Shinshu University, in collaboration with Waseda University, the Japan Fine Ceramics Center, and the University of Michigan (USA), announced the development of a separation membrane made of graphene-wrapped zeolite that can separate hydrogen at very high speed. The structure consists of zeolite crystals wrapped in graphene, which provides separation performance through a mechanism that differs from conventional ones. The research group was able to confirm that hydrogen and methane can be separated at a rate one hundred times faster than conventional separation. They have also confirmed the high separation performance of CO2. These findings are expected to contribute to the realization of a hydrogen energy society and were published in the May 18 issue of Science Advances.
Provided by Shinshu University
There are growing expectations for the use of hydrogen energy to realize a decarbonized society, but currently about 95% of the world's hydrogen production is based on fossil fuel resources such as natural gas (methane). Producing hydrogen with this process mainly uses steam reforming, where hydrogen is recovered in a steam reforming reaction, but the concentration of recovered hydrogen is 40-70%, requiring the separation of unreacted methane (CH4) and hydrogen. Deep cold separation is the main separation method, but it requires energy for cooling. For this reason, it can be expected to be used with low energy costs. However, energy-saving high-speed separation membranes have not been realized because their separation speed is slow, and pressure is required to increase the speed.
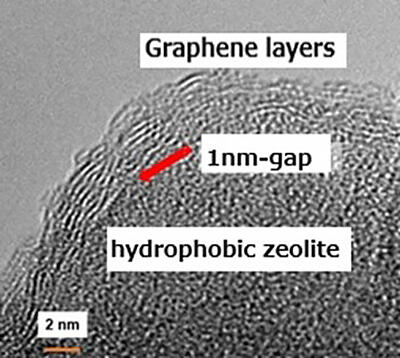
In their research, the group devised a separation membrane composed of graphene and zeolite. The separation membrane they developed uses the principles of colloid science, in which a hydrophobic zeolite (MFI-type zeolite crystal) is wrapped with a thin film of graphene, and the two are attached and encased by atomic forces. The graphene-wrapped zeolite is self-adhering and bonds closely together. Multiple 1-2 nanometer structural defects (nano windows) are created in the graphene.
The surface of zeolite crystals has structure-derived irregularities that create a gap of about 1 nanometer at the interface with graphene. Hydrogen molecules are about 0.3 nanometers in size and can pass through the interfaces and nano windows, while methane is about 0.4 nanometers in size and cannot pass through as easily as hydrogen. It is this property that can be used to filter the molecules.
The research group conducted a performance evaluation in cooperation with Waseda University. Graphene-wrapped zeolite was compressed at 18 kN to produce a separation membrane of approximately 6 mm square. They measured the transmission velocity of He, molecular hydrogen, CO2, N2, CH4, isobutane and sulfur hexafluoride gas. They then studied the permeability coefficient and selectivity of molecular hydrogen.
They confirmed the membrane had the property of being particularly selective in letting He and molecular hydrogen pass through. Furthermore, they found that the separation factor of hydrogen to methane is 245, while the permeation coefficient is 5.8 x 106 barrers, more than one hundred times higher than that of conventional polymer separation membranes.
The group performed dozens of isolation tests and did not identify any deficiencies. It is thought that by changing the crystals used for the material, it is possible to separate other molecules.
"We are currently engaged in research and development to separate oxygen, nitrogen, argon, and other elements in the air and use them as resources," says Professor Kaneko. "The target is mainly oxygen, which is different from this project, but if the separation membrane can be made large enough, it can be used to separate large amounts of oxygen, and we believe it can be applied to this separation as well."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




