A research group led by Associate Professor Takane Imaoka, Professor Kimihisa Yamamoto and graduate students Minori Inazu and Yuji Akada of the Laboratory for Chemistry and Life Science, Institute of Innovative Research at Tokyo Institute of Technology, has observed the bonding of different metal atoms in real time using a high-resolution electron microscope for the first time in the world. Using this technique, the team also succeeded in directly observing the smallest three-element alloy--gold (Au), silver (Ag) and copper (Cu)--containing one atom of each metal, which has been impossible until now. Their finding is expected to lead to the demystification of catalytic mechanisms and new designs. The study was published in the online edition of Nature Communications.
"Dynamic hetero-metallic bondings visualized by sequential atom imaging"
Licensed under CC-BY4.0
In recent years, it has become possible to directly observe atomic dynamics through continuous atomic image observation. Among the atomic bond arrangements seen one after another, many structures with sequences and bond distances consistent with calculated chemistry have been observed. However, elemental identification of a single atom is required to observe heteroatomic bonds with this method.
EDS (energy dispersive X-ray spectroscopy) and EELS (electron energy loss spectroscopy) are the most common analytical methods that can be combined with electron microscopy to identify elements, but neither method can be applied to identifying moving atoms, and the only way to determine the arrangement of each element has been to predict it using molecular orbital calculations.
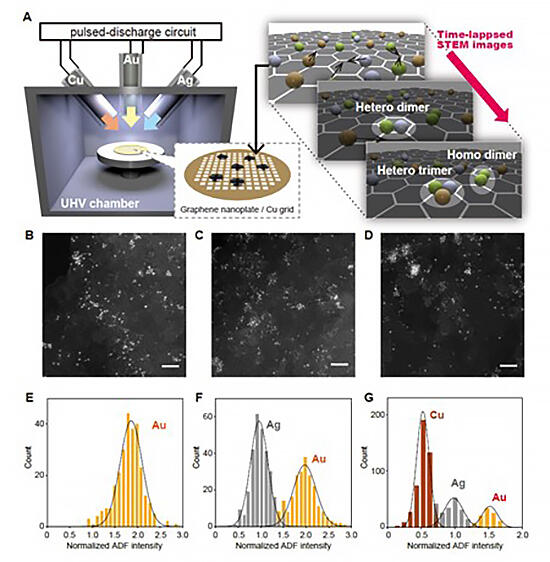
In their study, the research group employed annular dark-field scanning transmission electron microscopy (ADF-STEM), in which the brightness of a single atom correlates with its atomic number, as an observation technique in the structural analysis of the assembly of such multi-element metal molecules. Since atoms move moment to moment during observation, they conducted a video tracking analysis to measure luminance while tracking the coordinates of the atoms.
In ADF-STEM imaging, atoms are observed as bright spots, and their brightness is proportional to the nth power of the atomic number (n being a number between 1 and 2). They observed a large number of gold, silver and copper atoms deposited on graphene, and took atomic brightness distributions of the images with the appropriate noise and background removed. Utilizing the Z-contrast principle, they found each atom has a near-normal distribution, and each mode to be proportional to the 1.15 power of the atomic number Z. These results are in good agreement with the correlations predicted from the simulations. Also, the errors in the brightness of each atom obtained from the video tracking analysis were sufficiently small to discriminate gold, silver, and copper atoms from each other with more than 99% accuracy.
When the gold, silver and copper atoms deposited on graphene were observed in by video, the research group saw the atoms constantly move around the graphene surface, bonding and separating from each other. When atoms approach each other at a certain distance, they attract each other and appear to bond. To understand the pattern of movement, they examined statistics on the distance between two gold atoms in one video frame and the frequency of whether the bond stretches or shrinks in the next frame. In most cases, the distance between the two atoms was distributed with a frequency of about 0.26 nanometers, which is the bonding distance of gold dimers, and the frequency of growth and contraction switched around that distance. This indicates that an attractive force is acting between the atoms when they are farther than the equilibrium inter-nuclear distance (0.26 nanometers).
In addition, they observed bonds to be constantly formed and broken between atoms. Bonds were formed not only between atoms of the same metal but also between atoms of different metals, and they directly observed a total of 24 types of homonuclear and heteronuclear diatomic molecules for the first time. Furthermore, for the first time in the world, the group succeeded in observing triatomic molecules consisting of one atom each of gold, silver and copper, which are coinage metals that normally do not phase separate to form uniform alloys in bulk metals.
This method is expected to contribute to the structural identification of inorganic materials with amorphous structures such as metal sub-nanoparticles (clusters) in the future, leading to the design of new catalysts and other applications.
Journal Information
Publication: Nature Communications
Title: Dynamic hetero-metallic bondings visualized by sequential atom imaging
DOI: 10.1038/s41467-022-30533-y
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




