A research group led by adjunct principal investigators Hiroshi Kageyama and Ryu Abe (both professors at the Graduate School of Engineering) of the Institute for Cell-Material Sciences (iCeMS) at Kyoto University, in collaboration with the University of Tokyo, Tokyo Institute of Technology, National Institute of Standards and Technology (USA), Skolkovo Institute of Science and Technology (Russia) and University of Antwerp (Belgium) announced the successful synthesis of HSbOI, a new compound with positively charged oxide clusters. They confirmed its excellent catalytic performance as a solid acid catalyst. Most conventional oxide clusters have a negative charge, so this finding is expected to lead to the development of new functional materials. Their findings were published in the June 17 issue of Science Advances.
Provided by iCeMS
Polyoxometalates (POMs) are anionic metal oxide clusters consisting of interconnected metal-oxygen polyhedral units and were first synthesized in the 19th century. They have been studied as functional materials due to their catalytic properties, and optical and biochemical applications. Nearly all of these oxide clusters share the property of having negative charges.
Professor Kageyama and Professor Abe previously discovered in 2016 that a complex anion compound (Bi4NbO8Cl), in which oxygen and chlorine coexist, is an excellent semiconductor catalyst for splitting water into hydrogen and oxygen with visible light. Since then, with the aim of further improving photocatalytic activity, they have been investigating the synthesis of complex anionic compounds containing iodine, which has a smaller band gap due to its lower electronegativity than chlorine and can use a longer wavelength of visible light.
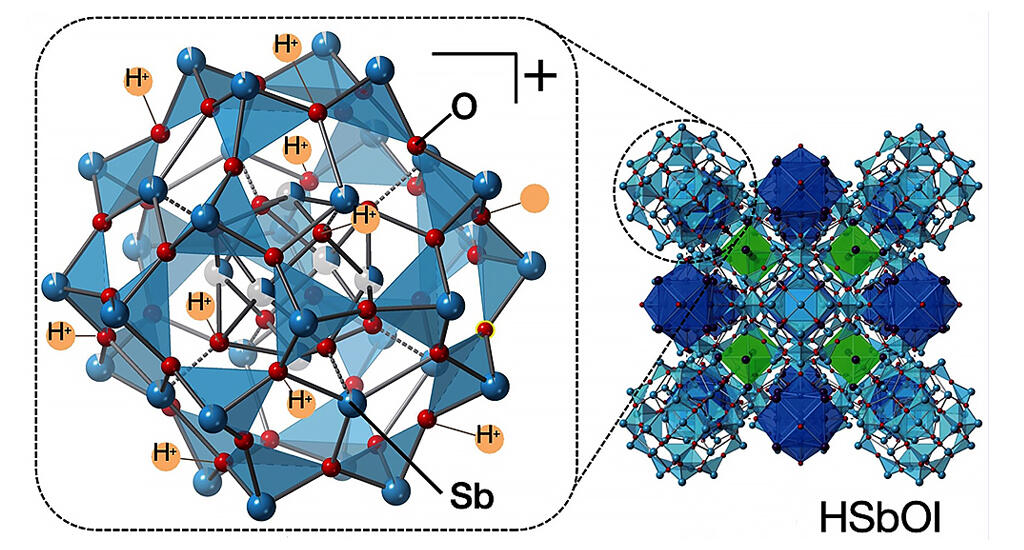
The compound synthesized in this study was examined and found to be HSbOI, a new substance composed of hydrogen, antimony, oxygen and iodine. They then combined three-dimensional electron diffraction tomography with synchrotron X-ray diffraction to conduct a structural analysis.
They found a giant cluster crystal composed of large, medium and small clusters, each containing about 800 atoms in the unit cell. The large clusters also had a positive charge and very weak binding of protons (H+) on their surfaces, suggesting high reactivity.
In order to investigate the possibility of using the high reactivity as a solid acid catalyst, they examined the pinacol rearrangement reaction, a typical acid-catalyzed reaction. The catalyst was found to have high catalytic performance as a solid acid catalyst. In addition, they recorded a high catalyst turnover number of over 300 times in trials of organic synthesis reactions using water as a solvent. The new material can be synthesized with a very simple process of dissolving antimony oxide (Sb2O3) powder in a solution consisting of hydrogen iodide (HI) and water to control the pH.
"Since the new material is not porous and the reaction occurs only on the crystal surface, we expect that its activity will be enhanced by combining it with other molecules and other substances," explains Dr. Kageyama. "Currently, theoretical calculations show that the medium clusters are unstable, so we think that we can simply change the negatively charged clusters and molecules that are combined with the large positively charged clusters. In conventional negatively charged oxide clusters, an infinite variety of substances have been synthesized by changing the positively charged ions and molecules with which they are combined." He adds, "We believe that applying the same method will improve stability. The new substance is currently in a solid state, but if we can stabilize it in a solution, we believe it will have a wider range of applications."
Journal Information
Publication: Science Advances
Title: Polyoxocationic antimony oxide cluster with acidic protons
DOI: 10.1126/sciadv.abm5379
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




