The research group of Associate Professor Kazuhiro Abe of the Cellular and Structural Physiology Institute at the University of Nagoya, researcher Christoph Gerle of RaQualia Pharma, the University of Southern Denmark, and the RIKEN SPring-8 Center, and researcher Hideki Shigematsu (same organization at the time of the research), in collaborative research, succeeded in a multiple analysis of a structure in which a gastric acid inhibitor is bound to a gastric proton pump (a membrane protein that is responsible for the secretion of gastric acid). This result clarified how existing drugs act on proteins at the molecular level.
Provided by Nagoya University
When digesting food, the stomach is filled with hydrochloric acid (HCl) and becomes strongly acidic (pH1). The gastric proton pump is a membrane protein on the surface of the stomach that transports gastric acid (H+) from the intracellular body into the stomach, and, because of its function, it has become a drug discovery target for the treatment of gastric acid-related diseases (gastric ulcers, reflux esophagitis, etc.).
X-ray crystal structure analysis methods and a cryo-electron microscope were used to analyze structures in which gastric acid inhibitors (which inhibit the action of gastric proton pumps) and analogous compounds were bound, and this analysis clarified how the gastric acid inhibitors are bound to the proteins. In addition, by clarifying the binding structure of multiple drugs, it will be possible to improve existing drugs and logically design new gastric acid inhibitors.
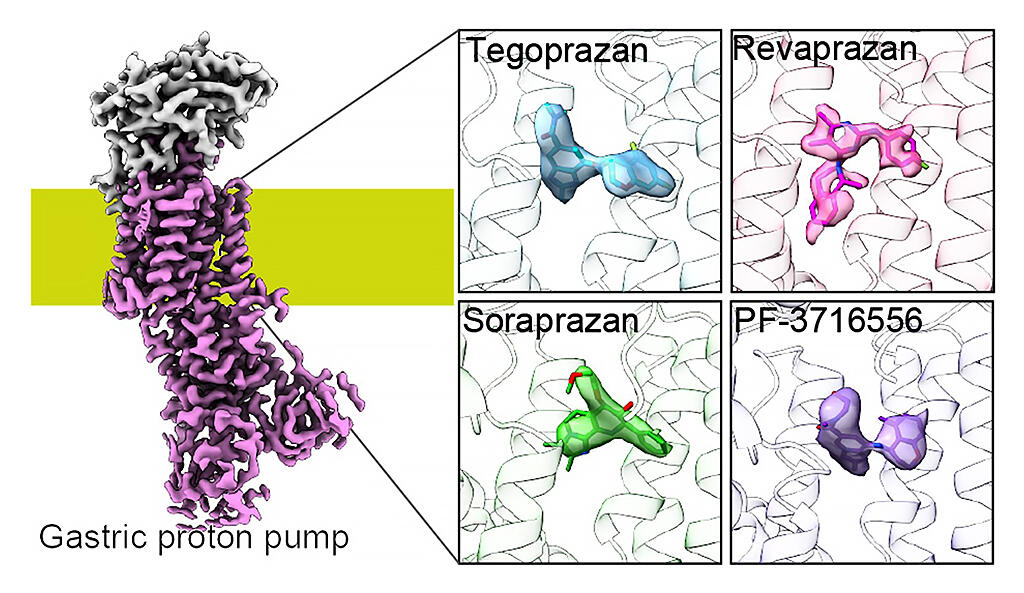
The research group conducted single particle analysis via a cryo-electron microscope and X-ray crystal structure analysis on the structures in which four different compounds (tegoprazan, soraprazan, PF-03716556, and revaprazan (tegoprazan and revaprazan are clinically used in Asian countries)) are bound. This analysis looked at the binding state at the amino acid level, and further verified the results via functional analysis using variants.
Of these, only revaprazan did not yield good crystals for analysis, so revaprazan's bound structure was obtained with high resolution through structural analysis via a cryo-electron microscope that does not require crystallization.
According to Associate Professor Abe, "We clarified numerous inhibitor binding structures, and we accumulated information on which parts of the binding pocket these compounds rely on to bind. Going forward, by improving existing drugs and designing new compounds, we would like to use this structural information to help patients who are suffering from gastric acid-related diseases."
Journal Information
Publication: Journal of Medicinal Chemistry
Title: Structural Basis for Binding of Potassium-Competitive Acid Blockers to the Gastric Proton Pump
DOI: 10.1021/acs.jmedchem.2c00338
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




