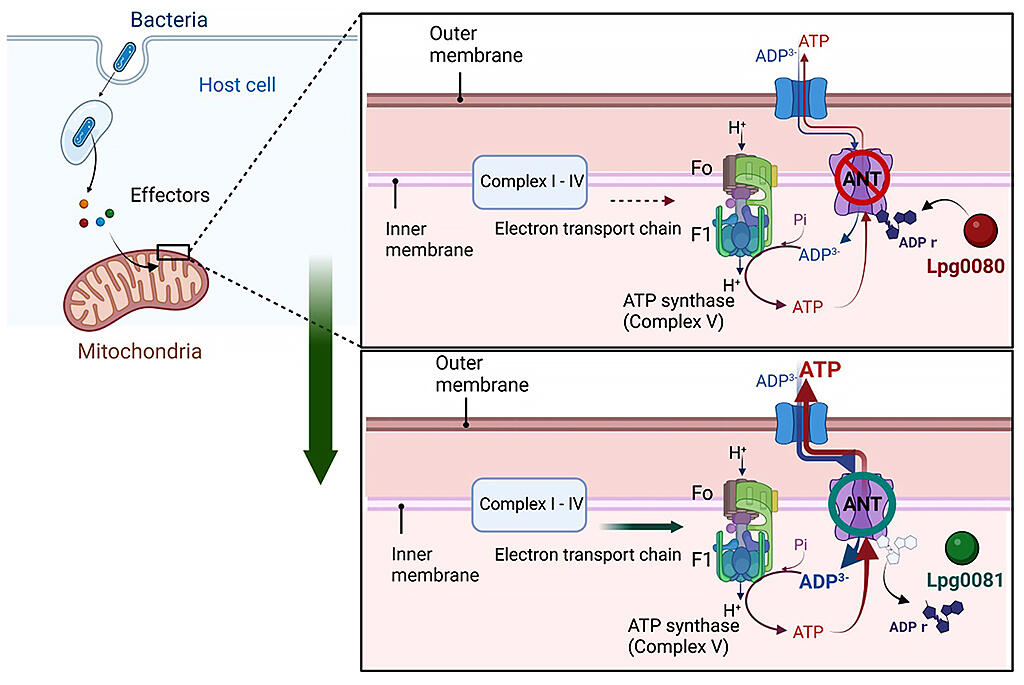
A research group consisting of Professor Hiroki Nagai, Associate Professor Tomoko Kubori and their colleagues in Microbiology in the Graduate School of Medicine, Gifu University has discovered that the bacterial pathogen Legionella, which causes serious pneumonia, regulates mitochondrial functions in host eukaryotic cells. Their research indicated that this regulation is based on a mechanism involving two Legionella enzymes that have been transported to the host cell working as a pair to induce the reversible chemical modification of mitochondrial ADP/ATP translocases. Moreover, through joint research with Professor Byung-ha Oh's research group from the Korea Advanced Institute of Science & Technology (KAIST), they have also clarified the detailed molecular structure of the modifying enzyme and its mechanism of action.
(The image was created with BioRender.com.)
Mitochondria, known as the powerhouse of the cell, form networks within the cells of the human body and are vital to maintaining life, but it is also known that when they are infected by pathogenic bacteria, their functions deteriorate. However, this mechanism was a mystery.
The research group found that Legionella, a bacterial pathogen that causes serious pneumonia, uses the activity of enzymes with functional proteins known as effectors to regulate mitochondrial function. They learned that one of these Legionella effectors, Lpg0080, causes the chemical modification of the ADP/ATP translocases (ANTs) that serve as pumps that transport ADP and ATP into and out of mitochondria, and thus puts the brakes on the action of the mitochondrial electron transport chain, including ATP synthase, the activity of which is linked to this. Moreover, it has become clear that Lpg0081, another effector, releases the brakes by undoing the chemical modification of ANTs.
Associate Professor Kubori commented, 'We discovered that the bacteria uses its own proteins (enzymes) to put the brakes on mitochondrial functions by causing the chemical modification of the mitochondrial energy transport pump. We also learned that these brakes are released through the activity of another bacterial enzyme. I believe that clarifying the mechanisms through which pathogens such as bacteria cleverly manipulate cell functions will provide a new perspective on drug discovery and development for treating and protecting against infections.'
■ ADP/ATP translocases: These exist in large quantities in the mitochondrial inner membrane. They are proteins that act to carry ADP, a material for ATP synthesis, from the cytoplasm to the inner part of the mitochondria, and also carry ATP synthesized in the mitochondria back to the cytoplasm.
Journal Information
Publication: PNAS
Title: Reversible modification of mitochondrial ADP/ATP translocases by paired Legionella effector proteins
DOI: 10.1073/pnas.2122872119
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




