A research group comprising Professor Shigeki Kiyonaka of the Graduate School of Engineering, Nagoya University、Professor Itaru Hamachi, Graduate School of Engineering, Kyoto University and Professor Michisuke Yuzaki, School of Medicine, Keio University has announced that, together with members of Kyoto University and Keio University, has announced the development of a chemogenetics method that can selectively activate glutamate receptors. The group confirmed that introducing a mutation in the type-1 metabotropic glutamate receptor (mGlu1), which functions in the neural circuits of the cerebellum that support motor functions and motor learning, causes it to respond to artificial ligands and activate, while the response function toward glutamate, which is essentially a ligand, is retained. It is hoped that their research results will lead to the clarification of the functions of different types of neuron and the development of a treatment method for diseases associated with loss of nerve activity. Their results were published in the June 16 edition of Nature Communications, an international science journal.
Provided by Nagoya University
Large numbers of neurons in the brain form neural circuits with complex connections, and it is through these that higher brain functions are created. Researchers believe that it is necessary to clarify the functions of the multiple types of neuron to understand brain functions; however, although the different types of neuron each have their own functions in the brain, they transmit information via multiple common receptors. Thus, it was difficult to clarify functions, even when using activation agents for each receptor.
Accordingly, in recent years, researchers have developed techniques such as optogenetics and chemogenetics, which selectively activate target cell types. On the other hand, as these control cell activity by forcing the expression of artificial proteins, it was not possible to analyze actual neurotransmission in the brain. Optogenetics is able to manipulate cell activity using light, by ensuring the genetic induction of light response proteins, but this requires physically embedding optical fibers in the brain, which is highly invasive, and cannot be applied to cells deep in the brain where light doesn't reach. Moreover, chemogenetics makes it possible to manipulate cell activity by expressing artificial receptors in cells that are selectively activated through artificial ligands that do not act on proteins inside the body, but the position of the receptors differs from their proper position.
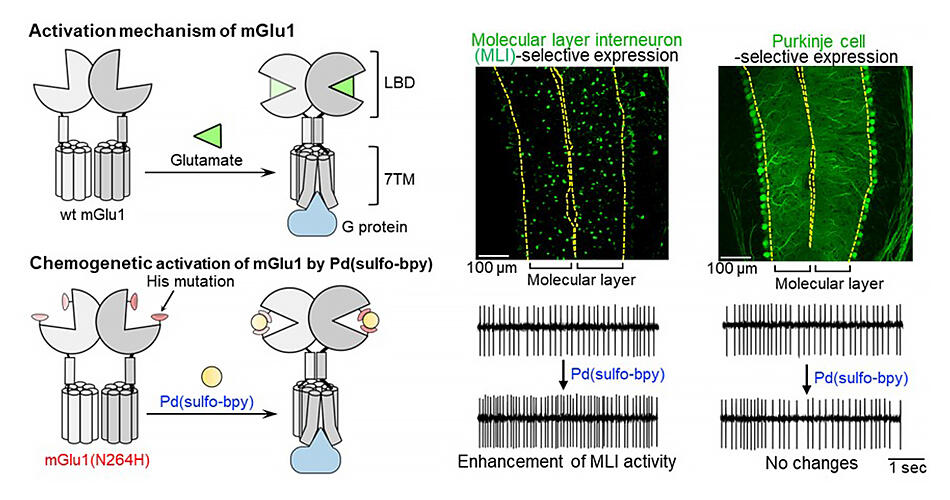
With this in mind, the research group focused on mGlu1, which is abundantly expressed in multiple types of neuron, especially in the cerebellum, which is responsible for motor functions and motor learning. They devised coordinated chemogenetics, a technique that selectively activates mGlu1 through artificial ligands, with the aim of clarifying the functions of each cell type. mGlu1 has glutamate binding sites on the outside of its cells, and researchers are aware that once it binds with glutamate (a natural ligand), information is transmitted within the cell through structural changes to this site.
Thus, to induce this structural change via artificial ligand binding, the group introduced mutations to glutamate binding sites and searched for appropriate artificial ligands. More specifically, they introduced histidine (metal-coordination amino acid) mutations to the entrance to glutamate binding sites, and discovered palladium bipyridyl complex (Pd (bpy)) as an appropriate artificial ligand. When the artificial ligands were administered to the mGlu1 mutation, the group confirmed that the original glutamate response was maintained, while there was also a response to the artificial ligand.
Moreover, the group assessed the cytotoxicity of the artificial ligands and designed Pd (sulfo-bpy) as an artificial ligand that has no effect on cell proliferation and neurite outgrowth.
When the group created knock-in mice with this mutation through genome editing (CRISPR/Cas9), the mice's motor functions, etc. were normal. They confirmed that cerebellar long-term depression (continuous weakening of the synaptic current response) was caused in conjunction with the normal activation of mGlu1 when Pd (sulfo-bpy) was added to cerebellar slices from these mice. They did not confirm this phenomenon when the same artificial ligand was added to wild-type mice.
In the cerebellum, mGlu1 is expressed not only in Purkinje cells, but also in granule cells and molecular layer interneurons (MLI) that regulate the functions of Purkinje cells. In the past, it was reported that in MLI, when the mGlu1 activation agent (DHPG) was added to a cerebellar slice, neural activity increased. On the other hand, researchers did not know which neuron types this increase was important for activating.
Thus, making use of the technique they developed, the group used adeno-associated viruses and cell-type-specific gene expression promotors to express the mGlu1 mutant in both Purkinje cells and MLI in mouse brains. Pd (sulfo-bpy) was administered to cerebellar slices from these mice.
While the results confirmed a neural activity increase in MLI, it also became clear for the first time that there was no increase in the Purkinje cells, and that the MLI activity increase depended on the activation of mGlu1 expression in MLI. It seems possible to apply this technique to other glutamate receptors, not just mGlu1, and it is hoped that it will be used to accelerate the clarification of higher brain functions associated with glutamate receptors, such as memory and learning, and will serve as a treatment method for neural diseases that are caused by an imbalance of these receptors.
Professor Kiyonaka commented, 'mGlu1 is thought to be important to motor learning. In the future, we hope to explore the phenotypes that appear in mouse actions, and how motor learning progresses, by adding artificial ligands to mice that express the mGlu1 mutant in Purkinje cells. I believe that understanding which brain circuits control our actions will be next-generation neuroscience.'
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




