The ability to extract hydrogen and oxygen from water using solar electricity would be a significant step toward achieving a carbon-neutral society. Achieving the high solar-to-hydrogen conversion efficiency (STH) necessary for this requires the development of high-efficiency water electrolysis cells operating at low overvoltages combined with solar cells with a maximum output suitable for these cells.
A research group led by Specially Appointed Assistant Professor Dr. Yuta Tsubonochi, Specially Appointed Associate Professor Dr. Zaki Nabeih Ahmed Zahran, and Professor Dr. Masayuki Yagi from the school of Science and Technology, Niigata University, in collaboration with a research group led by Dr. Kazuhiro Sayama, Dr. Takeyoshi Sugaya, Dr. Yugo Miseki, and Dr. Kikuo Makita of the Global Zero Emission Research Center, National Institute of Advanced Industrial Science and Technology, has developed a green hydrogen production system using solar-based electrolysis. The system consists of high-efficiency water electrolysis cells and solar cells. The groups have demonstrated that this system can stably produce hydrogen for one month at the world's highest STH (13.9%). The results were published in ACS Applied Energy Materials.
Provided by Niigata University with modifications by JST
To achieve high STH in a system of this kind, it is also essential to optimize their matching factor (MF) in addition to improving the efficiency of solar cells (STE) and water electrolysis cells (ETH).
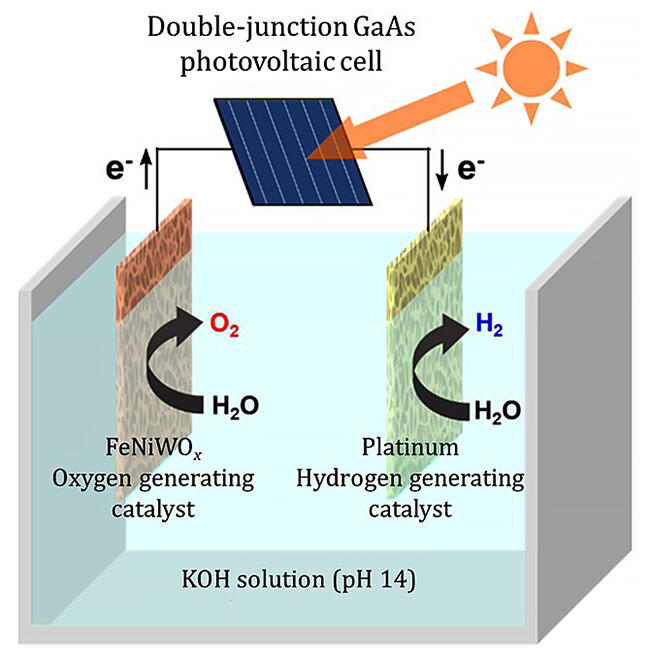
In 2021, the research group found that a mixed metal oxide containing iron, nickel, and tungsten (FeNiWOx) acts as a highly active and stable catalyst for oxygen generation. The groups fabricated a cell with a FeNiWOx electrode as an oxygen-generating anode combined with a platinum hydrogen generating cathode to electrolyze water at a lower overvoltage (240 millivolts) than a conventional electrolysis cell (around 315 millivolts).
Gallium arsenide (GaAs) solar cells are also known to be stable and exhibit high STE, but their electromotive force was insufficient for water electrolysis. However, the electrolysis cell the group developed operates at low overvoltages, so it can electrolyze water even with the electromotive force of a double-junction GaAs cell.
In fact, solar electrolysis under sunlight (1 sun) irradiation conditions using a double-junction GaAs solar cell and a water electrolysis cell demonstrated the ability to stably produce hydrogen from water over one month at the world's highest STH of 13.9% (the latest STHs are 6.1 to 16%). This high STH is achieved thanks to the high electrolysis efficiency of the water electrolysis cell (ETH 85%) and the optimal matching of the water electrolysis cell and the solar cell (MF 99%).
The research groups plan to develop a solar electrolysis system with an improved water electrolysis cell and solar cell, aiming for an STH of over 25%.
Journal Information
Publication: ACS Applied Energy Materials
Title: Perfect Matching Factor between a Customized Double-Junction GaAs Photovoltaic Device and an Electrolyzer for Efficient Solar Water Splitting
DOI: 10.1021/acsaem.2c00768
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




