A research group comprised of Assistant Professor Shohei Tada and Assistant Professor Tatsuya Joutsuka of the Graduate School of Science and Engineering, Ibaraki University, Senior Researcher Tetsuo Honma of the Japan Synchrotron Radiation Research Institute (JASRI), and Lecturer Kenta Iyoki of the School of Engineering, the University of Tokyo, has investigated the effect of Zn content on the hydrogenation reaction that converts carbon dioxide (CO2) to methanol with high efficiency using zinc zirconia catalysts and found the structure of the active site through experiments and calculations. The group found that when the level of zinc (Zn) content is low, Zn-O-Zr sites are formed on the catalyst surface and exhibit specific catalytic activity. They also successfully explained the mechanism by which Zn is unevenly distributed throughout ZrO2 when an excessive amount is added based on the stability of the crystal structure.
Provided by Ibaraki University
There is a need for technology to use carbon dioxide effectively in order to achieve carbon neutrality. One effective method is to generate methanol via a hydrogenation reaction from hydrogen and carbon dioxide, and use that methanol as the raw material to produce hydrocarbons and other materials. Zinc-zirconia catalysts, involving zinc dissolved in zirconia, have attracted much focus in recent years as a catalyst for methanol synthesis, but the effects and structure of the quantity of zinc introduced in the catalyst were not fully understood.
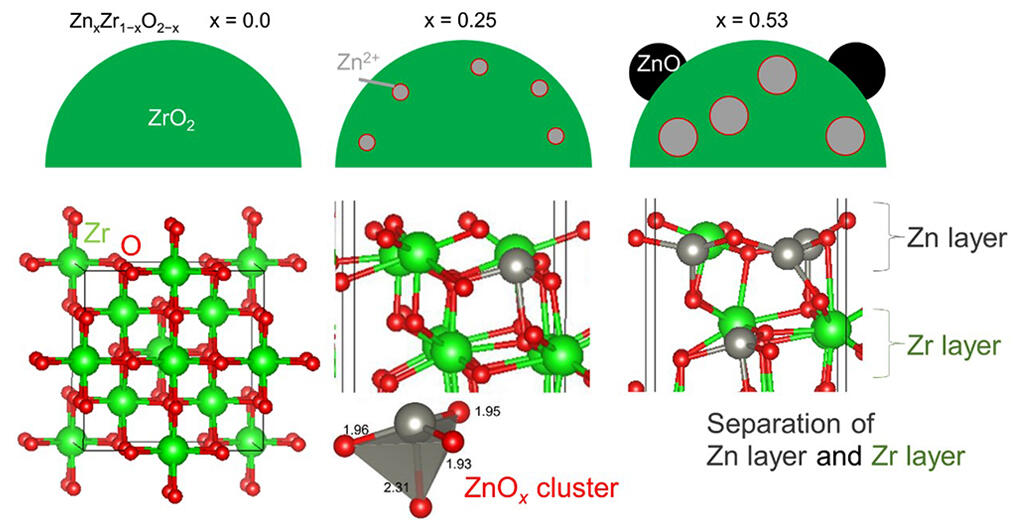
To address this, the research group used a fixed-bed reactor to analyze the catalytic performance of zinc-zirconia. When they increased the quantity of Zn in the zinc-zirconia, they found that the space time yield was at its highest when the quantity of zinc was x=0.25.
The group then examined the relationship between zinc quantity and catalyst structure using DFT calculations, and found that when the Zn quantity was low, Zn was readily exposed on the zinc-zirconia surface, forming Zn-O-Zr sites. Further analysis of the zinc-zirconia at the atomic level using an electron microscope and surface analyzer showed that cluster regions containing Zn were distributed unevenly near the zirconia (ZrO2) surface. However, they found that when the quantity of Zn was high, ZnO nanoparticles were formed in addition to the clusters containing Zn. Integrating the results of structural analysis and tests to evaluate catalytic performance, the group found that ZnO nanoparticles are not involved in the methanol synthesis reaction but that the performance of the methanol synthesis was determined by the number of Zn-O-Zr sites.
From the results of the DFT calculations, they also found that the adsorption of the reactant hydrogen (H2) becomes stronger when the quantity of zinc is increased, while the adsorption of carbon dioxide weakens at the same time. These results demonstrate that the Zn sites in the catalyst play an important role in hydrogen adsorption, while the zirconium (Zr) sites play an important role in carbon dioxide activation.
According to Iyoki, "By combining theoretical calculations with measurements made using a synchrotron radiation facility, we were able to gain insight into the way the structure and reaction mechanism changes depending on the amount of zinc introduced. We hope to leverage these findings in creating a new system for catalyst design, particularly combining it with a catalyst that converts to methanol."
■ Space time yield: the volume of target substance generated per unit of catalyst and time when the reactant passes through the catalyst bed.
■ DFT calculations: Computer simulations of electronic states based on density functional theory.
Journal Information
Publication: ACS Catalysis
Title: Active Sites on ZnxZr1-xO2-x Solid Solution Catalysts for CO2-to-Methanol Hydrogenation
DOI: 10.1021/acscatal.2c01996
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




