The research group of graduate student Yukako Oe of the Graduate School of Frontier Biosciences, Osaka University, Associate Professor Shuhei Nakamura and Professor Tamotsu Yoshimori of the Graduate School of Medicine, Osaka University, have succeeded in bringing to light a new mechanism that controls the fusion of the "amphisome" intermediate and the lysosomes formed in the process of autophagy.
Provided by Osaka University.
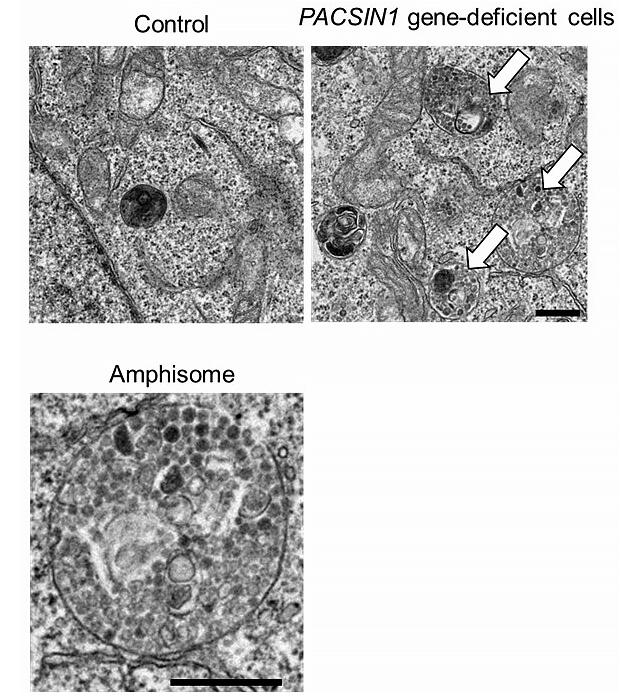
Autophagy is an intracellular degradation system commonly present in eukaryotes, and is known to be induced by starvation and stress. When autophagy is induced, various materials in the cytoplasm are surrounded by structures called autophagosomes and then transported for degradation to lysosomes containing digestive enzymes. In this process, it is known that the autophagosomes directly fuse with the lysosomes, or that they fuse with the lysosome after passing through an intermediate called an amphisome, but the control mechanisms for and the significance of these two pathways were unknown.
The research group found that basal autophagy activity was reduced in PACSIN1 gene-deficient cells, and, when they investigated the cause, found that the fusion process between lysosomes and amphisomes, which are an autophagosome/endosome hybrid structure, was inhibited. In addition, the reduction in autophagy activity that was observed in PACSIN1 gene-deficient cells was not observed in starvation-induced autophagy, which suggests that the two fusion pathways have different uses depending on either the stimulation of autophagy induction or substrate degradation.
Therefore, they investigated whether these pathways are used differently in selective autophagy, which specifically degrades certain degradation substrates. It was found that although PACSIN1 is required for lysophagy, which selectively removes damaged lysosomes, and for aggrephagy, which selectively removes protein aggregates, but that PACSIN1 is not required for mitophagy, which selectively removes defective mitochondria.
Furthermore, in order to clarify the physiological role of the amphisome-mediated fusion pathway, the research group used nematode and cultured mammalian cells to investigate accumulations of α-synuclein (a protein that is abundant in the central nervous system) aggregates. In contrast to control nematodes, PACSIN gene-deficient nematodes had enhanced accumulation of α-synuclein aggregates. Similar results were obtained from the experiments with cultured mammalian cells. This suggests that the amphisome-mediated autophagy pathway prevents the accumulation of protein aggregates.
Associate Professor Nakamura said, "While examining the function of PACSIN1, we found for the first time that the autophagy pathway is used differently depending on the degradation target. In particular, the PACSIN1-mediated amphisome pathway was found to play an important role in the degradation of aggregated proteins that cause neurodegenerative diseases. We hope that, in the future, these findings will lead to the establishment of new methods for prevention and treatment for neurodegenerative diseases."
Journal Information
Publication: PLOS Genetics
Title: PACSIN1 is indispensable for amphisome-lysosome fusion during basal autophagy and subsets of selective autophagy
DOI: 10.1371/journal.pgen.1010264
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




