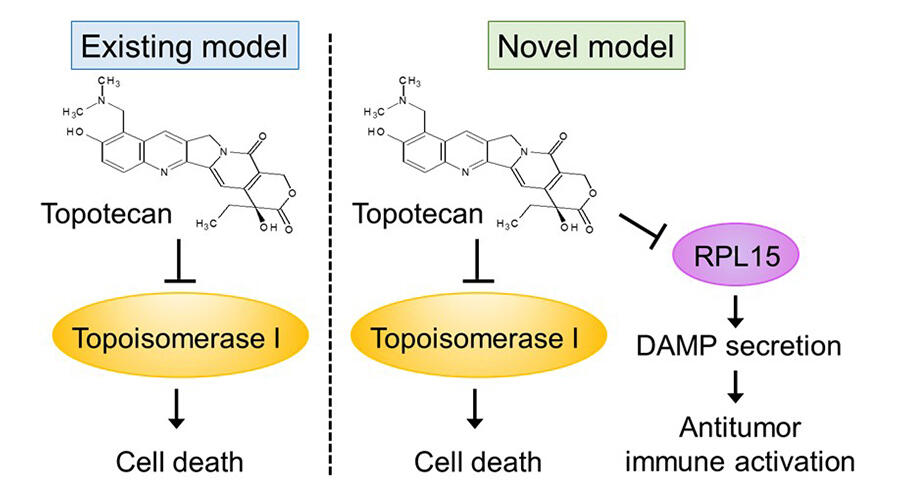
A research group consisting of Assistant Professor Yuichi Kitai and Professor Tadashi Matsuda of the Faculty of Pharmaceutical Sciences, Hokkaido University and their colleagues has clarified that topotecan, an anticancer drug, encourages antitumor immune activation by inhibiting RPL15, a novel target protein. Their results were published in the Journal of Immunology.
Provided by Hokkaido University
It is known that when cells incur different types of damage, for example due to chemical substances, radiation or temperature stress, they release molecules known as DAMP, which activate the immune system. As the release of DAMP by cancer cells encourages cancer immune activation and with this the destruction of cancer cells, researchers believe that it also affects the efficacy of cancer treatment.
Previously, the research group has reported DAMP, which activates the immune system, is released from cancer cells treated with topotecan, and encourages a cancer immune response. However, they did not understand the kind of mechanism that induced this phenomenon.
From this background, in order to explore novel topotecan target proteins, they collected topotecan binding proteins by creating beads bound to topotecan and mixed these with cell extract from cancer cells, ensuring the precipitation of the beads. By carefully analyzing the collected proteins, they identified RPL15 as a novel topotecan binding protein.
Next, to verify the correlation between RPL15 and DAMP release from cancer cells, they transplanted skin cancer (melanoma) cells with reduced RPL15 production resulting from knockdown into mice and assessed the composition and activation state of the immune cells infiltrating tumors. The results showed that the tumors with RPL15 knocked down saw significantly less growth than the controls.
Moreover, the group was not able to confirm any tumor suppressing effect due to anti-PD1 antibodies in the control tumors, but tumor growth was significantly reduced due to anti-PD1 antibodies in the RPL15 knockdown tumors. In addition, the results of flow cytometry analysis of the immune cells that infiltrated the tumors showed that cytotoxic T cells significantly increased in the RPL15 knockdown tumors, but, conversely, the regulatory T cells that regulate the cancer immune response decreased.
These results suggested that RPL15 inhibition causes the cancer microenvironment to change, becoming an environment in which cell damage due to cytotoxic T cells is easily induced, and that the tumor suppressing effect due to anti-PD1 antibodies increases. In the future, the group anticipate that, if it is possible to develop a specific inhibitor for RPL15 that does not act on topoisomerase I, this will contribute to improving cancer immunotherapy, including with anti-PD1 antibodies.
Journal Information
Publication: The Journal of Immunology
Title: Identification of RPL15 60S Ribosomal Protein as a Novel Topotecan Target Protein That Correlates with DAMP Secretion and Antitumor Immune Activation
DOI: 10.4049/jimmunol.2100963
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




