Deliquescence is the phenomenon by which a substance takes up water vapor in the air and spontaneously becomes an aqueous solution. A familiar example is the phenomenon by which table salt, which contains bittern (magnesium chloride), hardens as it takes up water vapor from the air. In comparison, the deliquescence phenomenon by which a substance takes up volatile organic compounds (VOCs) instead of water vapor to transform into a liquid has not yet been reported. A research group led by Professor Kazuyuki Ishii of the Institute of Industrial Science, The University of Tokyo, has become the first in the world to discover a unique phenomenon in which molecular salts exposed to VOCs gradually transform into liquid. A systematic study has proven that the phenomenon is in fact deliquescence triggered by VOCs, which the group has dubbed organic deliquescence.
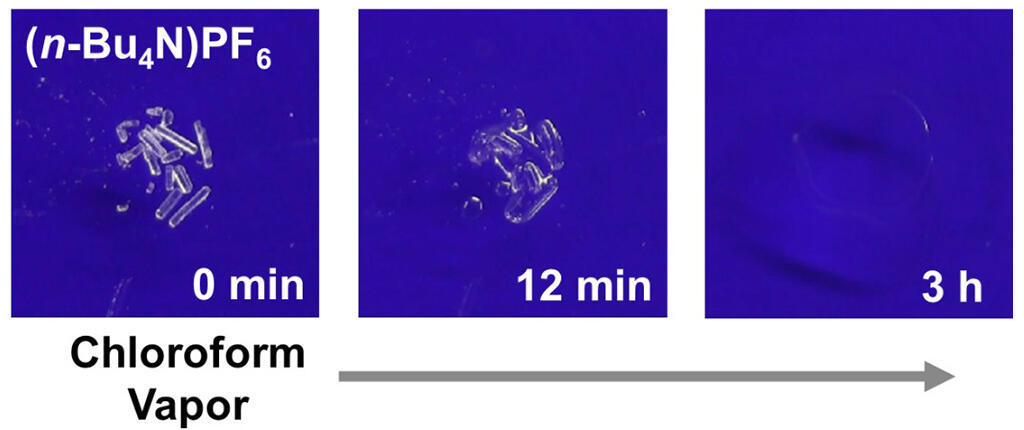
Source: The University of Tokyo, RSC Adv., 2022, 12, 18307-18310. with modifications.
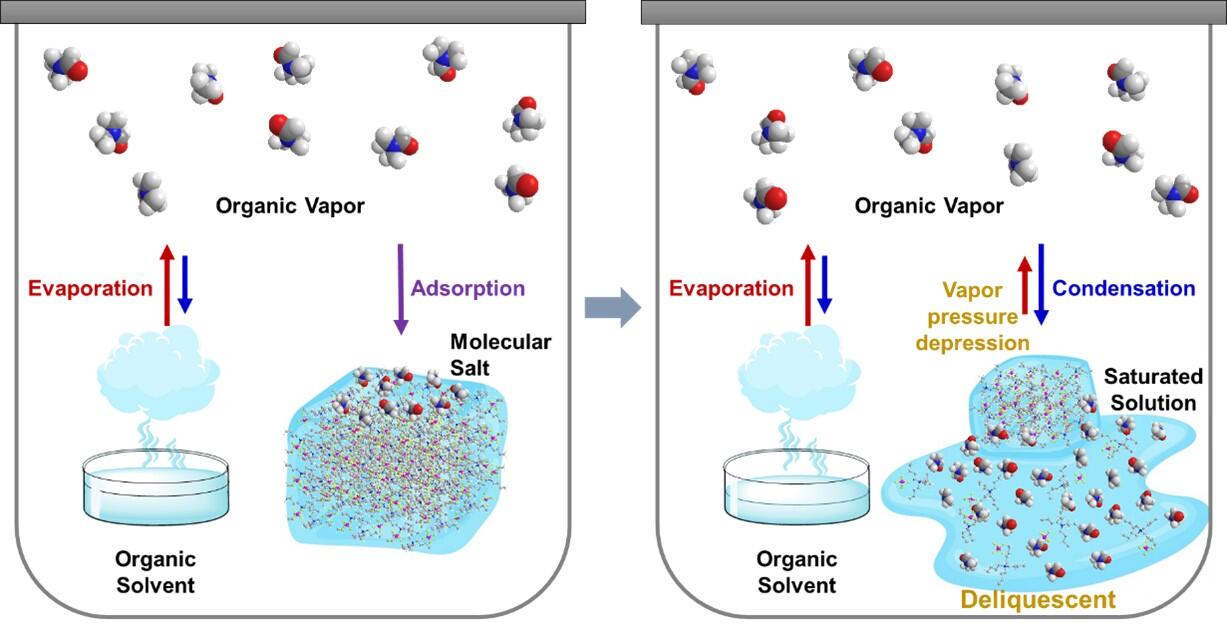
Source: The University of Tokyo, RSC Adv., 2022, 12, 18307-18310. with modifications.
In the experiment, compound crystals and powder were placed in containers, while a separate organic solvent was added at a distance, and the container was sealed. Subsequently, state changes in the first substance were tracked after exposure to the VOCs. As a result, the group observed the phenomenon by which the compound powder gradually transformed into a liquid as the VOCs were depleted. The group confirmed that there was an increase in mass during the transformation from powder to liquid, demonstrating that a deliquescence phenomenon had occurred triggered by the VOCs.
The group observed organic deliquescence when using chloroform, a solvent widely used in the chemical industry, as the VOC, and tetrabutylammonium hexafluorophosphate ((n-Bu4N)PF6) as the salt, but not with such salts as ammonium hexafluorophosphate (NH4PF6), ammonium tetrafluoroborate (NH4BF4), and sodium chloride (NaCl).
When the VOC used was high polarity dimethylformamide (DMF), a compound used in large quantities in the wet spinning of textiles, and capable of dissolving a variety of salts, organic deliquescence was not observed with NaCl, but it was observed with (n-Bu4N)PF6, NH4PF6, and NH4BF4. After the organic deliquescence of those salts, the mass of the solvent greatly exceeded that of the salts, demonstrating an important characteristic of organic deliquescence compared to conventional adsorption. The organic deliquescence phenomenon was also observed in the non-ammonium salt tetraphenylphosphonium bromide ((Ph4P)Br), demonstrating that it is a general phenomenon rather than one unique to ammonium salts.
According to Professor Ishii, "This organic deliquescence can be controlled according to the well-known principle in chemistry that similar substances dissolve well together. Considering that the dissolution phenomenon of calcium chloride is utilized in desiccants, I expect that the phenomenon of organic deliquescence can be applied to development of ways to recover VOCs, one of the causes of atmospheric pollution."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




