The National Institutes for Quantum Science and Technology (QST) has designed and built the world's first trailer-based radioisotope (RI) facility capable of targeted radioisotope therapy (TRT) using alpha ray-emitting Actinium-225 (225Ac). The concept was based around using an inexpensive, easy-to-install, mobile trailer-based facility, overturning the conventional wisdom of RI treatment facilities, which require expensive and time-consuming construction and installation. The Nuclear Regulation Authority approved its use as a controlled radiation area on June 30.
Provided by QST
225Ac is an RI that has attracted worldwide attention since a drug labeled with it was reported to show exceptionally high therapeutic efficacy against metastatic prostate cancer (Heidelberg University, Germany, 2016). To realize TRT using 225Ac-labeled drugs in Japan, researchers are developing methods that contribute to the domestic production of 225Ac and 225Ac-labeled drugs for mesothelioma and synovial sarcoma. However, a key issue is that it is difficult to secure places to conduct 225Ac-TRT in Japan, and there is concern that this will create future social challenges when the treatment becomes a reality.
Facilities that handle RI can only use RI in quantities (radioactivity) approved by the Nuclear Regulation Authority based on factors such as equipment capacity. Many existing TRT facilities are being assigned the maximum permitted use of pharmaceutically approved beta- and alpha-emitting RIs to maximize their ability to provide treatment. Obtaining new permits to use 225Ac would require reducing the use of RI for which permission has already been obtained.
However, this would significantly disadvantage patients, as they would no longer be able to receive the treatments currently being provided. New or expanded TRT facilities would need to be built to allow the use of the new 225Ac treatments while maintaining the amount of RI needed for pre-existing TRT facilities.
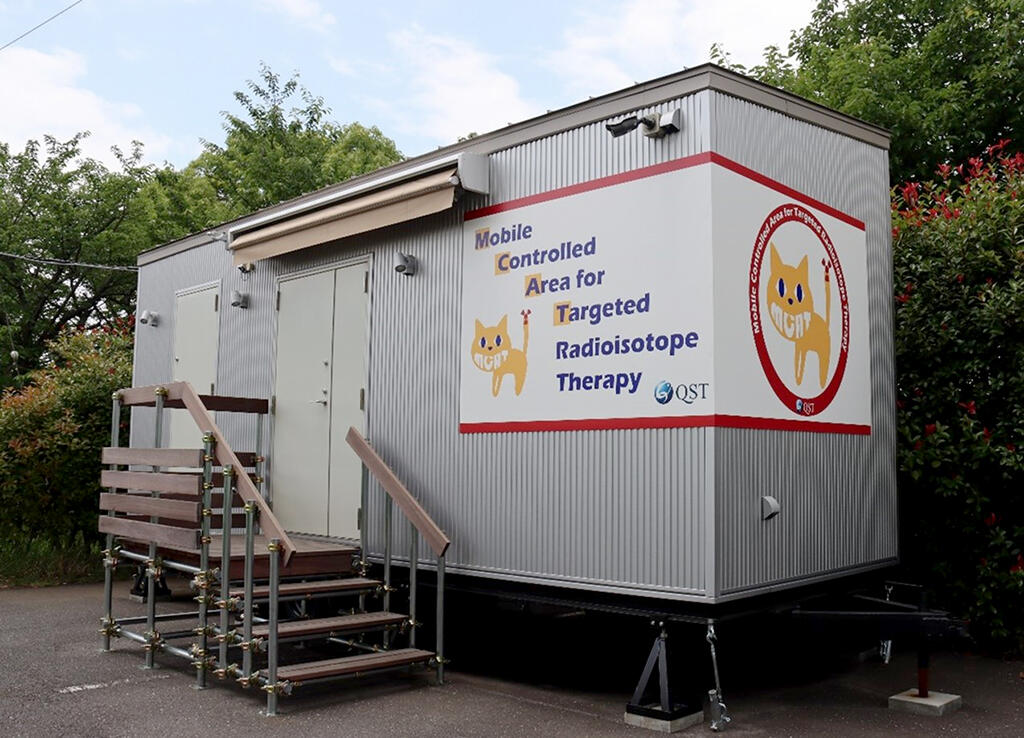
To deliver 225Ac, which is expected to have high therapeutic benefits, to as many patients as possible, QST devised a trailer-based RI facility that can be manufactured and installed at about one-tenth the cost of a new or expanded conventional RI facility (several hundred million yen) and can be moved because it complies with road traffic laws. The trailer is 2.35 meters wide, 7 meters long, and 3.75 meters high. Since it can easily be moved, it can temporarily be installed in areas such as hospital parking lots and is therefore expected to contribute significantly to the spread of 225Ac-TRT.
QST plans to conduct a first-in-human study for 225Ac-labeled drugs in three years. It also plans to conduct a demonstration study for clinical use by measuring airborne concentrations and air doses when 225Ac is used in trailer-based facilities and clinical simulation tests.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




