A research group led by Assistant Professor Hironobu Osaki and Professor Mariko Miyata of the Division of Neurophysiology, Department of Physiology, Tokyo Women's Medical University, used research on the somatosensory system of mice to clarify that the dysgranular region inside the primary somatosensory cortex (the somatosensory region of the cerebral cortex, which is the outermost part of the brain) play a key role in pain perception processing.
Provided by Tokyo Women's Medical University
When it comes to animals, the sense or sensation of how much something hurts objectively evaluates an animal's pain with pain-like behavior. According to Professor Miyata, "In the case of animals, we have to clarify whether avoidance behavior is really due to pain. That's because we also see behavior involving simply running away due to surprise." Thus, there is a need to analyze the relationship between the strength of a stimulus that causes an animal to feel pain and their behavior, and subtle animal actions such as the direction of escape - for example, do they run in the opposite direction from where a pain stimulus is coming?
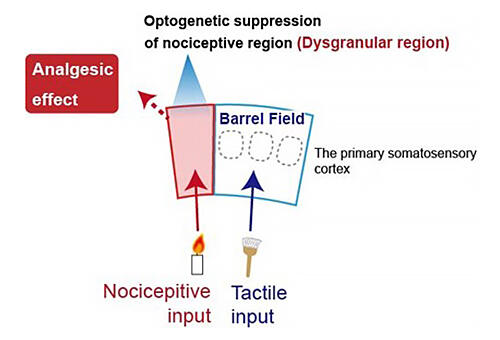
The barrel field, which responds tactile sensations, and the adjacent dysgranular region are located in the somatosensory cortex in the cerebral cortex. During this research, the group simultaneously recorded extensive neural activity from the barrel field and the dysgranular region. When they closely observed cell distribution for each characteristic of these responses, they found that in the 2/3 layer of the cerebral cortex (a shallow layer) that processes sensory information in the initial stages, many cells in the dysgranular region responded only to nociceptive stimuli, and many cells in the barrel field responded only to tactile stimuli. The group learned that the dysgranular region selectively responds to nociceptive stimuli in a highly sensitive manner, and that the barrel field is very responsive to tactile stimuli. Although these characteristics are maintained when it came to the cells of layer 5 (the layer that receives input from the 2/3 layer and outputs this to several different regions of the brain), there was a higher number of cells that responded to both nociceptive and tactile stimuli in the dysgranular region and the barrel field.
To verify whether the dysgranular region is associated with chronic pain such as neuralgia, the group ligated (ligation: to tie off a vessel or tube, so nothing passes through) the second branch of the trigeminal nerve (the infraorbital nerve), the sensory nerve of mice whiskers, with a surgical thread, creating a trigeminal neuralgia model. The outcomes made it clear that when the mice were in a state with similar symptoms to trigeminal neuralgia, the dysgranular region activated.
Moreover, to directly prove the causal relationship between dysgranularregion activity and pain behavior, the group used optogenetic techniques to alter neural activity by shining light only on the dysgranular region and investigating how pain behavior changed. They discovered that when a nociceptive stimulus was applied, dysgranular region activity was reduced, and the mouse no longer displayed previously seen pain behavior. Conversely, when this was increased, they found that the mouse displayed pain behavior in response to stimuli so weak no pain-like behavior was previously displayed.
Professor Miyata commented, "The dysgranular region is a small region that lies in the region known as the somatosensory cortex in the cerebral cortex. It is equivalent to the region known as the 3a area in humans' cerebral cortex, primary somatosensory cortex. As we were able to remove pain (relieve pain) by selectively inhibiting this region, we believe this may lead to the development of therapeutic techniques to remove pain (pain relief) by cleverly controlling 3a region activity in humans."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




