A joint research group led by Assistant Professor Daichi Kato, Professor Ryu Abe and Professor Hiroshi Kageyama of the Graduate School of Engineering, Kyoto University, Professor Masatomo Yashima of the Department of Chemistry, School of Science, Tokyo Institute of Technology, Professor Akinori Saeki of the Graduate School of Engineering, Osaka University, and Professor Hitoshi Takamura of the School of Engineering, Tohoku University, announced that they have discovered the ability to control the coexistence rock salt and fluorite structures, the most basic crystal structures in inorganic compounds. Their findings were published online in Advanced Functional Materials on August 2.
Provided by Kyoto University
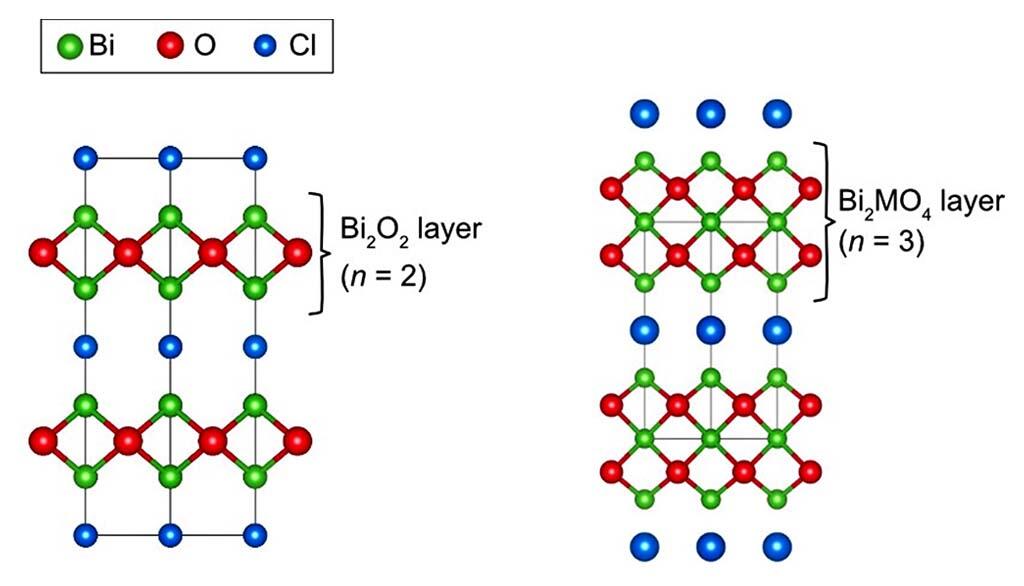
The rock salt structure represented by sodium chloride (NaCl) and the fluorite structure represented by calcium fluoride (CaF2) are the most basic crystal structures in inorganic compounds. There are also a number of known compounds with rock salt structure layers (rock salt layers) and fluorite structure layers (fluorite layers).
In this study, Assistant Professor Kato and his collaborating research group analyzed the structure of the acid chloride Bi12O17Cl2, a known photocatalyst, and found that it has a corrugated structure due to the inclusion of a rock salt unit partially within the fluorite layer. Furthermore, they conducted a reaction to insert fluorine into this, rearranging the rock salt and fluorite units and flattening the corrugated Bi12O17Cl2 layers, significantly increasing the photocatalytic activity by up to six times. Assistant Professor Kato and his colleagues believe that the ability to freely combine these two basic structures will lead to the development of new functional materials.
The joint research group focused on Bi12O17Cl2, a bismuth acid chloride. This substance has been studied intensively over the past five years because of its high photocatalytic activity for various applications, such as environmental purification and artificial photosynthesis. However, even today, its crystal structure remains a mystery nearly 40 years after its existence was first reported in the 1980s, which has prevented further improvement in the activity of this substance.
In their joint research, the group combined various analytical techniques such as electron microscopy, X-ray and neutron diffraction and single-crystal X-ray diffraction to reveal that Bi12O17Cl2 has a layered structure consisting of a Bi-O layer and a Cl layer, similar to other bismuth acid chlorides. Furthermore, they found that its Bi-O layer has two unique features compared to conventional bismuth acid chlorides.
The first feature is that the thickness of the Bi-O layer is n=6, making it the "thickest" Bi-O layer found to date. The second feature is that, unlike conventional acid chlorides, the Bi-O layer has a wavy structure due to the coexistence of rock salt within the Bi-O layer in addition to fluorite units. Furthermore, based on the knowledge of the crystal structure revealed in this study, they succeeded in synthesizing a new acid fluoride chloride by fluorine insertion reaction into Bi12O17Cl2 at low temperature.
Structural analysis revealed that in this new acid fluoride chloride, the corrugated structural distortion of the Bi-O block disappeared and was instead rearranged in the interlayer direction into alternating layers of fluorite and rock salt layers. They measured the photocatalytic activity for organic matter decomposition and found it to be greatly enhanced with fluorinated materials.
The research group believes that this is because the distortion of the corrugated structure had disappeared, and the electrons produced by the absorption of light flowed more easily through the layer.
Both rock-salt and fluorite structures are typical crystal structures, but there is no other example of compounds of two types of structural units in the same layer, which is fascinating from the viewpoints of structural chemistry and materials science. "In addition to its performance as a photocatalyst, the very unique crystal structure is the appealing point of this research," explains Assistant Professor Kato.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




