The research group of Specially Appointed Associate Professor Yuki Kobayashi, Specially Appointed Professor Hikaru Kobayashi, from the Institute of Scientific and Industrial Research (ISIR: SANKEN) at Osaka University, Yoshichika Yoshioka from the Graduate School of Frontier Biosciences at Osaka University, along with Assistant Professor Yoshihisa Koyama and Professor Shoichi Shimada, from the Graduate School of Medicine/Faculty of Medicine at Osaka University, in collaboration with Associate Professor Iwao Otsu of the Faculty of Life and Environmental Sciences at the University of Tsukuba, announced that they had confirmed in mice that a silicon agent improves the symptoms of ulcerative colitis. In a mouse model, the silicon agent suppressed inflammation-induced disruption of intestinal structures and systemic inflammation. The activation of nerve cells observed in the untreated group was also suppressed, suggesting the possibility that the silicon agent may also act to alleviate sensory and mental symptoms in the brain. The results are expected to lead to the development of therapeutic agents for ulcerative colitis, which is an intractable disease of unknown cause. These findings were published in the June 11 issue of Scientific Reports, an American scientific journal.
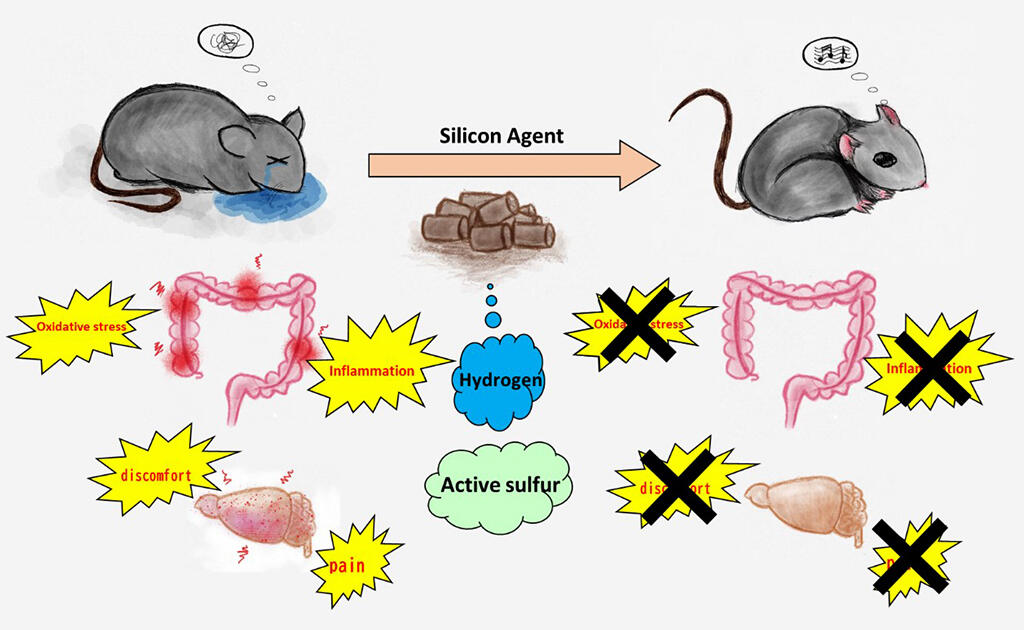
Provided by Yoshihisa Koyama, Osaka University
Ulcerative colitis is an intractable inflammatory bowel disease and it is estimated that there are more than 220,000 patients with it in Japan. In the disease, ulcers and erosions develop on the mucous membrane of the large intestine, and this inflammation continuously spreads, causing diarrhea, bloody stools, and abdominal pain, and, in severe cases, causes full-body symptoms such as fever. The disease is widespread in both young and elderly people and becomes chronic through repeated remissions and flare-ups. There are a variety of factors that cause recurrences, such as excessive labor and the accumulation of stress, and in order to maintain remissions it has been necessary to develop drugs that target not only the large intestine, but also the brain.
The research group had previously developed a silicon agent that, when taken orally, generates large amounts of hydrogen in the intestinal tract in a sustained manner, and confirmed the neuroprotective effects of the agent by administering it to a mouse model of Parkinson's disease.
In this study, the research group focused on hydroxyl radicals (a strong reactive oxygen species) that are constantly generated in the body, and, aiming to eliminate them, verified the effectiveness of the silicon agent as new an antioxidant. Reactive oxygen is neutralized in the body when people are healthy, but it increases due to disease and psychological stress, etc., causing an oxidative stress state that exacerbates aging and diseases. Abnormal accumulations of oxidative stress have also been reported in the intestines of patients with ulcerative colitis.
A DSS-induced (Dextran sulfate sodium) ulcerative colitis model was used as a mouse model. Assuming administration during a remission period, the research group set two groups, one that was fed a diet containing the silicon agent (2.5% silicon agent) (the treatment group) and one that was fed a normal diet (the control group), and each diet was started seven days prior to DSS administration. After DSS administration, follow-up observations, pathological analysis, and brain analysis, etc. were conducted.
As a result of the treatment, when the amount of hydrogen in the intestines was measured via gas chromatography, when compared to the control group, the treatment group showed a significant increase in hydrogen content and had significantly alleviation of various symptoms such as weight loss, diarrhea, and bloody stools. Furthermore, the treatment group showed significantly lower levels of inflammatory cytokines, which are markers of inflammation, and MRI analysis showed a reduction in inflamed areas around the intestinal tract. Additionally, the structural collapse of the large intestine that occurs in ulcerative colitis was significantly suppressed in the treatment group.
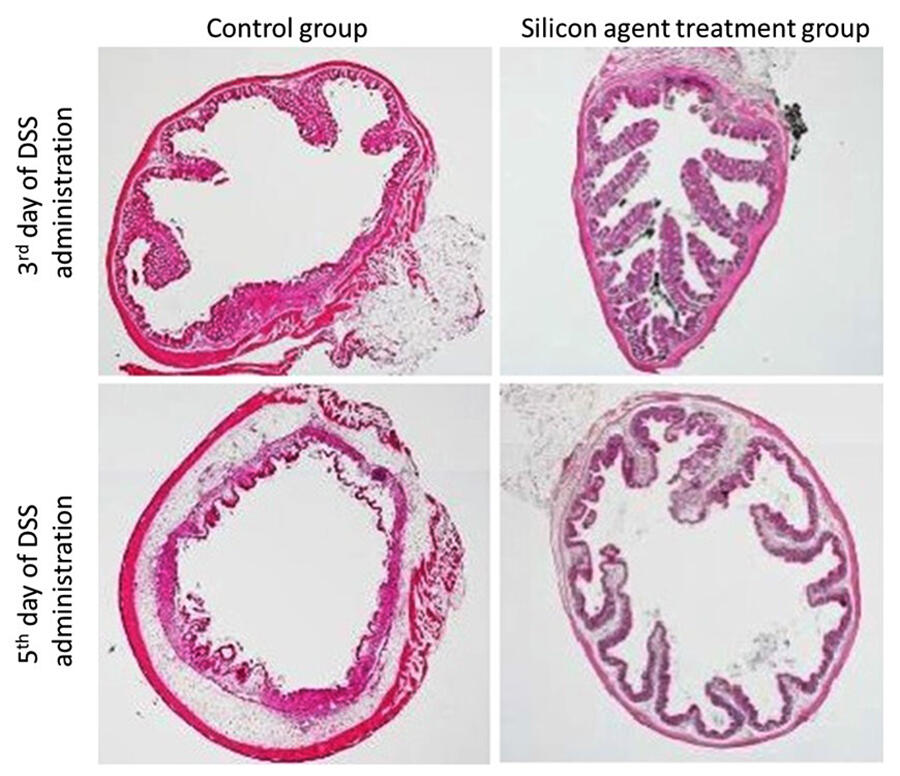
Provided by Yoshihisa Koyama, Osaka University
Whole-body oxidative stress levels were examined by measuring blood oxidative stress levels (dROMs), and until the third day of DSS administration there was no difference between the two groups, but on the fifth day a sharp increase was noted only in the control group. Sulfur index analysis (metabolic analysis of sulfur compounds) was performed to investigate the degree of oxidation in the large intestine itself, and it was found that active sulfur, which is a powerful in-vivo antioxidant, was significantly higher in the treatment group. Furthermore, in order to investigate the effects on sensory and neurological systems, the research group examined the activity of neurons in the dorsal medulla oblongata, which is involved in perceptions of internal organs and involuntary movements, and in the amygdala, which is involved in likes, dislikes, and pleasure.
After doing so these regions were activated in the control group. However, the treatment group showed reduced activation in the dorsal medulla oblongata and no activation in the amygdala, indicating that the neurological symptoms had been alleviated. It is thought that the pain and discomfort in internal organs was alleviated via the administration of the silicon agent.
Basic research to date has confirmed that the silicon agent that was developed is non-toxic, does not react in the acidic environment of the stomach but does react with water in the environment of the intestinal tract where pancreatic and intestinal juices are secreted at pH 8.3, and that a large number of hydrogen atoms are bound to the surface of the silicon agent during the reaction.
Hydrogen molecules are considered to have strong bonds between the hydrogen atoms and are unlikely to react with hydroxyl radicals, so it is very likely that the hydrogen atoms on the surface of the silicon agent greatly contributes to the elimination of hydroxyl radicals. It is thought that the reductivity of the hydrogen atoms generated on the surface of the agent leads to the generation of active sulfur.
Currently, the research group is aiming to apply for the silicon agent to be approved as a drug and are conducting physician-led clinical trials in cooperation with pharmaceutical companies. According to Assistant Professor Koyama, "We believe that the silicon agent can be a new drug candidate for ulcerative colitis remissions and that it can break the vicious cycle of flareups. To that end, we want to further advance our research on silicone agents."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




