The research group of Professor Takakazu Nakabayashi, Assistant Professor Shinya Tahara, and graduate student Kosuke Yamazaki of the Graduate School of Pharmaceutical Sciences at Tohoku University have brought to light a new molecular mechanism in which SOD1 toxicity acquisition is involved in the development of ALS (Amyotrophic Lateral Sclerosis).
Provided by Tohoku University
ALS is a progressive neurodegenerative disease, and the development of treatment methods has not progressed because the cause of its onset is unknown. Although aggregates of SOD1 (a metalloprotein that binds copper and zinc ions) are found in ALS lesions, the detailed mechanism of the relationship between SOD1 toxicity and ALS development was unclear.
The research group showed that, when the structure of SOD1 changes, it is not an antioxidant but rather has a highly toxic oxidative action that oxidizes surrounding compounds, and they also investigated the oxidation mechanism. In these results, the research group succeeded in showing for the first time that the antioxidative and oxidative actions of SOD1, which are completely opposite functions, are switched via the formation (antioxidative) and cleavage (oxidative) of a single sulfur-sulfur (disulfide) bond in SOD1.
It is said that oligomers of several molecules of SOD1, rather than aggregates of SOD1, are involved in the expression of SOD1 cytotoxicity. However, the research group clarified that the oligomers are formed when the sulfur-sulfur bond is cleaved, and that the oligomers exhibit strong oxidizing action. This allowed them to propose that the toxicity of SOD1 oligomers derives from their oxidative action.
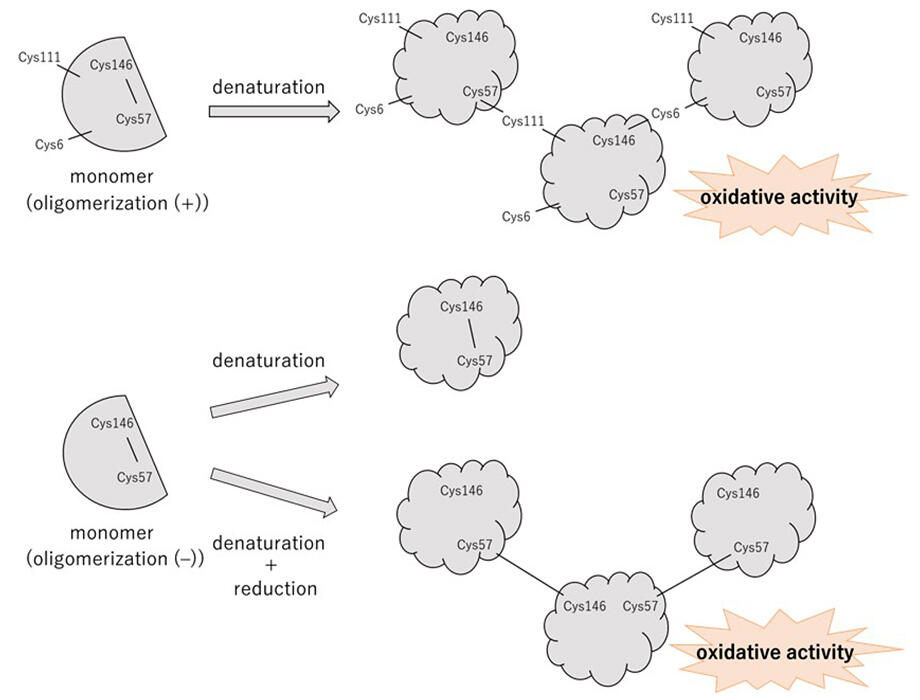
To test this, the research group prepared variants without the ability to form oligomers. SOD1 forms oligomers via intermolecular disulfide bonds between cysteine residues (Cys). Four Cys are present in SOD1, with Cys6 and Cys111 existing as reduced free thiol groups (-SH groups) and Cys57 and Cys146 forming intramolecular disulfide bonds.
Therefore, for Cys6 and Cys111, the research group prepared a non-oligomerizing variant by substituting the free Cys to eliminate its ability to form intermolecular disulfide bonds. The relationship between oxidative activity and oligomerization upon thermal denaturation was examined by using the normal, oligomer-forming normal SOD1 and the non-oligomer-forming variant. Oxidative action was quantified both before and after thermal denaturation via the use of the DCF fluorescence method, and, in ordinary SOD1 that is capable of forming oligomers, the thermal denaturation resulted in the formation of oligomers and an increase in oxidative action. On the other hand, the variants in which the free Cys was substituted showed no oligomerization and no oxidative action.
These results indicate that the toxicity of oligomers is from the oxidative action produced by the cleavage of intramolecular disulfide bonds that are associated with oligomer formation. Acquiring the oxidative action does not require thermal denaturation or oligomerization, but occurs solely via cleavage of the intramolecular disulfide bond. SOD1 was shown to switch the enzymatic activity of the body-protecting antioxidant action to the bad oxidative action only via the cleavage of intramolecular disulfide bonds.
Professor Nakabayashi said that, "This research has shown that cleavage of the intramolecular disulfide bonds in SOD1 changes its enzymatic activity from an antioxidative action to an oxidative action. Targeting intramolecular disulfide bonds has a basic scientific potential for the development of both therapeutic and preventative drugs."
■ DCF fluorescence method: A method for evaluating the amount of reactive oxygen species that are generated by using the fluorescence intensity of dichlorofluorescein (DCF) molecules.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




