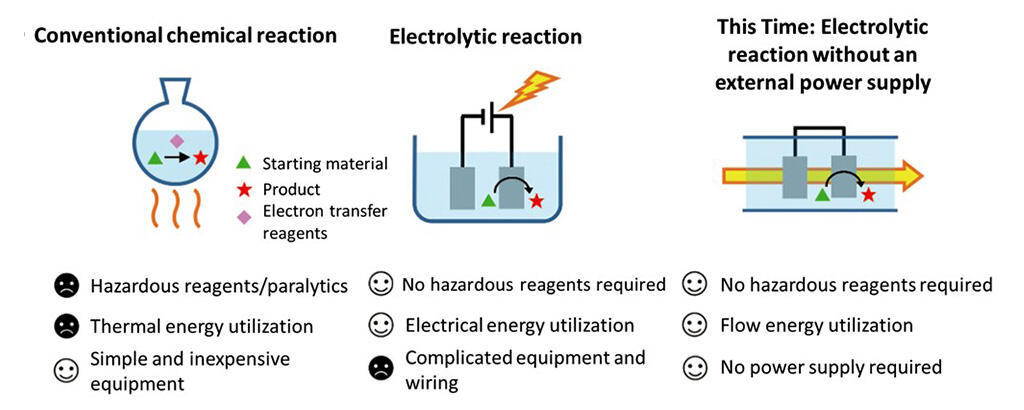
Attempts are being made worldwide to replace chemical reactions that use hazardous reagents with safe electrolytic reactions that have low environmental impacts. Realizing this aim would help solve environmental and energy problems. In electrolytic reactions, the substrate comes into contact with a charged electrode, with the reaction proceeding by exchanging electrons. These reactions reduce the types and amounts of reagents used compared to conventional methods, and typically no energy from heat is required. However, the time and effort required to install a power supply unit to power the electrodes, along with the hassle of wiring, created significant barriers.
Professor Shinsuke Inagi and colleagues at the Department of Chemical Science and Engineering, part of the School of Materials and Chemical Technology at the Tokyo Institute of Technology, have developed an electrolytic reaction technology that does not require an external power supply.
The group first focused on a phenomenon where a difference in potential is generated when a dilute electrolyte is fed into microchannels. It then examined various organic solvent and electrolyte combinations and concentrations, including the materials of the microchannel, to generate a difference in potential of about 3 volts, which is sufficient for an electrolytic reaction.
Electropolymerization was carried out using a flow of aromatic compounds, resulting in the formation of conductive polymer films. This finding suggests that an electrode placed upstream or downstream of the flow path became an anode, and an oxidation reaction proceeded.
The results show that even with various solvent and electrolyte combinations, electrolytic reactions can be harnessed simply by flowing the electrolyte through the microchannels. Currently, the system requires a high-pressure flow, but the group is working on improvements to enable efficient reactions even at low pressures. In the future, it is hoped this technique can be applied to electrolytic reactions such as fine chemical synthesis and decomposition of hazardous substances and see use in extreme environments such as the deep sea or areas under high pressure.