When searching for targets, mosquitoes sense carbon dioxide, human scent, and body heat. A research group led by Professor Makoto Tominaga of the Division of Cell Signaling, National Institute for Physiological Sciences (NIPS), has identified amino acid residues important for determining the activation temperature of TRPA1, a temperature and pain receptor in mosquitoes.
Many animals, including mosquitoes, sense temperature using TRPA1, a type of temperature-sensitive TRP channel. TRPA1 is a receptor for wasabi in humans and is known to sense temperature and pain stimuli. Tominaga's group had previously compared mosquitoes from different habitats, finding that the lowest temperature at which TRPA1 is activated (temperature threshold) varies widely by habitat. For example, in mosquitoes from warmer, tropical habitats, TRPA1 is activated at temperatures 8 to 10°C higher than in mosquitoes from relatively cool climates. Mosquitoes from the tropics are thought to have higher temperature thresholds to prevent environmental temperatures from being perceived as a painful stimulus. However, the mechanism behind this difference was unclear.
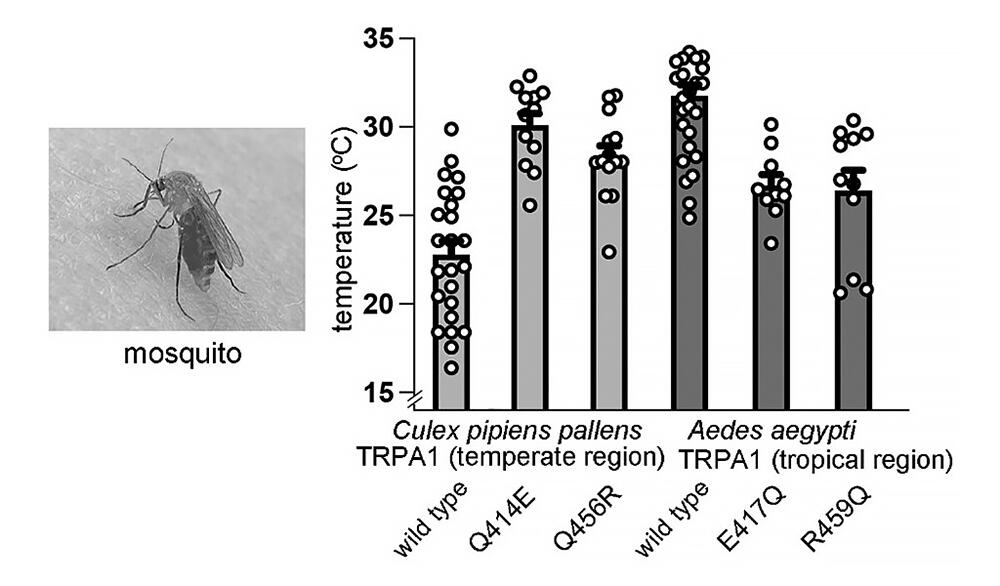
To determine which sections of TRPA1 are important for thermoreception, the group first conducted an experiment in which they artificially replaced some of the amino sequences in two varieties of mosquito: Aedes aegypti (Aa, Yellow fever mosquito) and Culex pipiens pallens (Cp, Common house mosquito). The team switched the TRPA1 amino terminal domains in tropical Aa, which has TRPA1 that activates at relatively higher temperatures, with those from the temperate Cp, which has TRPA1 that activates at relatively lower temperatures. When only the TRPA1 amino terminals were from Aa and all other amino sequences were from Cp, activation was only seen in warmer temperatures as with Aa TRPA1. However, when only the amino terminals were taken from Cp, and the rest was TRPA1 from Aa, activation was only seen at lower temperatures as with Cp's TRPA1.
These results indicate that the amino acid terminal domains are vital structures for determining the activation temperature threshold. A more detailed functional investigation revealed that the 70 amino acids at the amino terminals are essential for determining the activation temperature threshold. After creating and analyzing point mutants of several amino acids that differ from Aa's TRPA1 and Cp's TRPA1 among the 70 amino acids, the team found that two charged amino acid residues (acidic glutamic acid E and basic arginine R) are important in Aa's version of TRPA1. Another tropical mosquito, Anopheles stephensi (As, Asian malaria mosquito), was also found to have the same two amino acids as Aa. Replacing those two amino acids with Cp amino acids (both glutamine Q) lowered its activation temperature threshold.
Provided by National Institute for Physiological Sciences
Professor Tominaga said, "We found that two charged amino acid residues at the amino terminal domain of mosquito TRPA1 are important in determining the temperature threshold for activation. We believe that salt bridges (weak ionic interactions between side chains) between acidic and basic amino acids stabilize the protein's three-dimensional structure, making it less susceptible to conformational changes in response to thermal stimuli. We hope our results can help elucidate the mechanism by which temperature-sensitive TRP channels sense temperature."
Journal Information
Publication: Journal of Biological Chemistry
Title: Single amino acids set apparent temperature thresholds for heat-evoked activation of mosquito transient receptor potential channel TRPA1
DOI: 10.1016/j.jbc.2022.102271
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




