In Japan, approximately 25% of the population is said to be obese and approximately 0.05% is said to be morbidly obese, and there are concerns that these numbers will further increase with the spread of remote working. Obesity causes diabetes, heart attacks, strokes, and other health problems, and, because there is no fundamental treatment other than weight loss, it is hoped that new treatment methods will be developed to eliminate obesity.
A research team led by Part-time Lecturer Masakazu Fuji, Assistant Professor Daiki Setoyama and Professor Emeritus Dongchon Kang of the Department of Clinical Chemistry and Laboratory Medicine, Graduate School of Medical Sciences, Kyushu University, investigated the mechanism that activates brown fat cells (brown adipose tissue), which burn fat and produce heat, and brought to light a new mechanism that enables sustained fat burning by secreting and utilizing factors that promote activation in the brown fat cells themselves. The results were published in iScience.
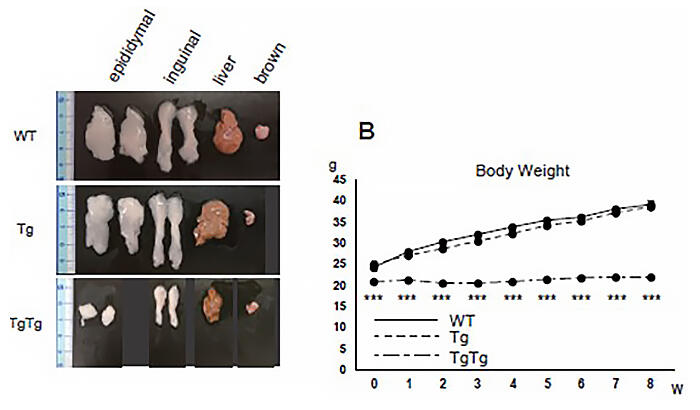
Provided by Kyushu University
The research group focused on TgTg (high TFAM expression) mice, which display a strong anti-obesity effect via the overexpression of a protein called TFAM (mitochondrial transcription factor A) that exists in mitochondria and examined the details of the activation mechanism for the brown fat cells that are notably activated in these mice, with the aim of applying their findings to the development of curative obesity drugs.
They found that the brown fat cells in TgTg mice had enhanced mitochondrial function when compared to wild-type (WT) mice, and, along with this, the TgTg mice also had elevated expression of the proteins required for the activation of brown fat cells. Next, to confirm that the brown fat cells derived from TgTg mice themselves exert an anti-obesity effect, brown fat cells from TgTg mice were extracted, cultured, and subcutaneously transplanted near the brown fat tissue of WT mice, and the researchers found that weight gain in the WT mice was suppressed even when they were raised in high-fat conditions.
That is, when the TgTg-derived and WT-derived brown fat cells were co-cultured in a culture medium, even the WT-derived brown fat cells were activated. Interestingly, in the TgTg-derived brown fat cells, it was confirmed that the extracellular secretion of exosomes was significantly increased due to their enhanced mitochondrial function. In other words, the research team revealed for the first time a new mechanism for activating brown fat cells, in which these over-secreted exosomes reach the WT-derived cells through the culture medium and contribute to their activation.
This clarification of the mechanism in which brown fat cells are activated by exosomes, which are physiologically present in the body, will provide a new therapeutic strategy that meets the important obesity treatment requirements of both safety and efficacy. In the future, the establishment of a mitochondrial activator that contributes to promoting the secretion of extracellular vesicles such as exosomes and the establishment of the stable collection of exosomes will greatly contribute to the development of curative obesity treatments.
Journal Information
Publication: iScience
Title: TFAM expression in brown adipocytes confers obesity resistance by secreting extracellular vesicles that promote self-activation
DOI: 10.1016/j.isci.2022.104889
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




