A research group led by Professor Hisako Sato of the Graduate School of Science and Engineering, Ehime University, in collaboration with Toho University, Nihon University, and Nagoya University, has succeeded in identifying the adsorption structure of a monovalent iridium complex intercalated in montmorillonite clay using their independently developed vibrational circular dichroism (VCD) method.
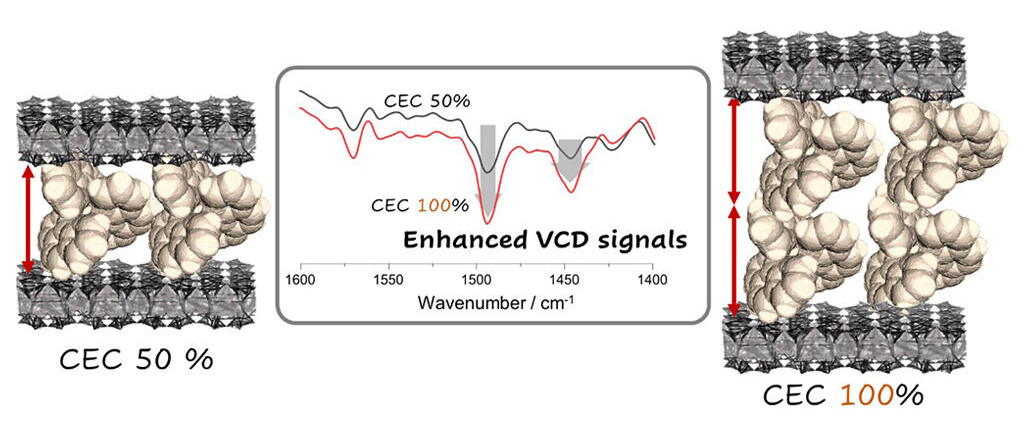
VCD for molecular interaction. Molecular packing in intercalation states was reflected on the relative intensity of a VCD signal. Enantiopure monovalent iridium(III) complexes formed mono- or bi-molecular layers in the interlayer spaces of montmorillonite (clay) at 50 and 100 % CEC (cation exchange capacity) loading, respectively. Solid state vibrational circular dichroism (VCD) spectra were applied to investigate the intermolecular interactions within adsorption layers. VCD signals were revealed to be sensitive enough to reflect the detailed features in the molecular packing of adsorbed molecules.
Provided by Professor Hisako Sato, Graduate School of Science and Engineering, Ehime University
Clay minerals are layered inorganic compounds that can incorporate a variety of molecules and ions between their layers. Due to these properties, clay is widely used in fields such as slow-release drugs, cosmetics, removal of toxic ingredients such as caffeine, and as a catalyst carrier. When chemical reactions occur in the interlayer space of clay, it is conceivable that the two-dimensionally restricted space is an ideal site for molecular recognition (identifying each other's shape and properties) because of the close proximity and adsorption of reactant molecules.
However, it is quite difficult to identify how they actually interact. The reason is that clay minerals are insulating microcrystals (less than 1μm), which precludes the use of X-ray structural analysis or probe microscopes such as tunneling microscopes, which are effective at the molecular level, so the identification of the molecular recognition mechanism on clay surfaces at the micro level has been an unsolved problem for quite some time.
The research group applied VCD to ionic molecules incorporated in clay mineral interlayer spaces. VCD is usually used for solution samples, but the signal is weak. In order to eliminate false signals caused by anisotropy inherent to solid samples, they improved the instrument and optimized the measurement method. In the study, the enantiomers (optical counterparts) of a monovalent tris-chelate complex (benzo[h]quinoline) were used to investigate interactions between adsorbed molecules. The results show that the VCD spectra are sensitive to the effects of intermolecular interactions. Theoretical calculations also allowed the research group to identify in detail the differences in intermolecular interactions between single-molecule and bimolecular layers. The iridium complexes used in this study have been the focus of attention in photoreactions, and are anticipated to be developed into heterogeneous catalytic reactions using clay minerals as supports in the future.
"The iridium complexes used in our experiments are compounds that have attracted attention for their high luminescence and photo reactivity," explains Professor Sato. "By combining these complexes with inexpensive and environmentally friendly clay minerals, we would like to expand our research into the construction of highly selective heterogeneous photo-asymmetric catalytic reaction systems and high-energy intensive systems."
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




