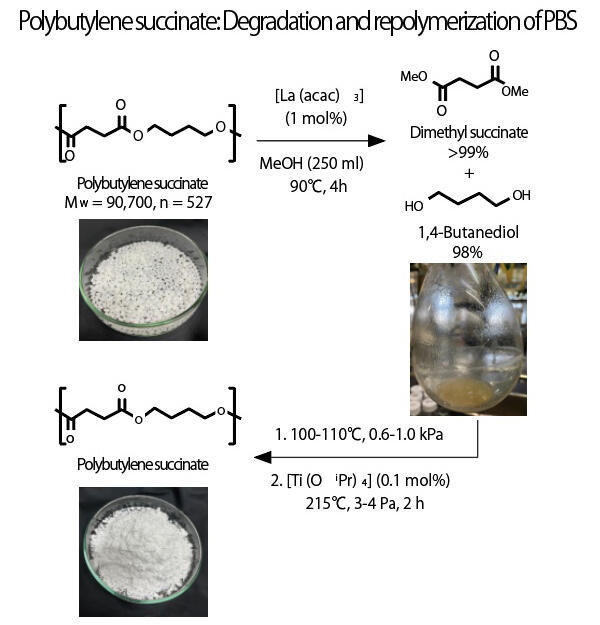
Plastic waste is one of the major social issues facing the world. Although the recycling rate for plastics in Japan is 86%, most of it is reused as fuel, and only about 3% is recycled as chemicals, which are reused as raw materials. Polyesters used in commercial PET bottles and other products have a repeating ester structure, which is synthesized from a monomer with carboxylic acids at both ends and a monomer with alcohols at both ends. However, chemical recycling is not widespread because it requires chemical reactions using bases and additives, which have a high environmental impact.
A research team led by Professor Kotohiro Nomura of the Graduate School of Science, Tokyo Metropolitan University, graduate student Ryota Abe of the Graduate School of Engineering, Tokyo University of Agriculture and Technology, and Assistant Professor Nobuyuki Komine and Professor Masafumi Hirano of the Department of Applied Chemistry, Faculty of Engineering, Tokyo University of Agriculture and Technology, have discovered that complexes of the rare earth element lanthanum are effective in decomposing polybutylene succinate (PBS) based on their knowledge of the transesterification decomposition of vegetable oils and the catalytic bond scission of esters of low-molecular-weight compounds. In their study, not only was PBS quantitatively degraded at a reaction temperature of 90°C, over a reaction time of four hours, but they also confirmed that commercial PET bottles were completely degraded and returned to raw materials. Moving forward, the team will identify catalytically active species with a view to social implementation and aim to construct more economical degradation reactions under mild conditions, as well as to create more valuable chemicals than plastics before degradation.