A research group led by Junior Associate Professor Masaaki Sadakiyo of the Department of Applied Chemistry, Faculty of Science 1, Tokyo University of Science, and graduate student (at the time) Yuto Yoshida of the Department of Chemistry, Graduate School of Science, Tokyo University of Science, in collaboration with Professor Teppei Yamada at the Department of Chemistry, School of Science, the University of Tokyo, Professor Ken-ichi Shimizu and Assistant Professor Takashi Toriyao of the Institute of Catalysis, Hokkaido University, has successfully developed a new solid magnesium ionic conductor that exhibits the world's highest ionic conductivity, achieving an ionic conductivity of about 10-3 S per square centimeter at room temperature, which is the practical ionic conductivity required as an electrolyte for rechargeable batteries.
Provided by Tokyo University of Science
Conductors that propagate magnesium ions can enable storage of cheap, renewable energy and their application in magnesium ion-based batteries is expected in the future. However, magnesium ions are divalent, and are known to propagate less easily in solids than monovalent ions. The research group has been studying the possibility that divalent magnesium ions can also be propagated efficiently if the pores in porous materials are used as ionic conduction pathways.
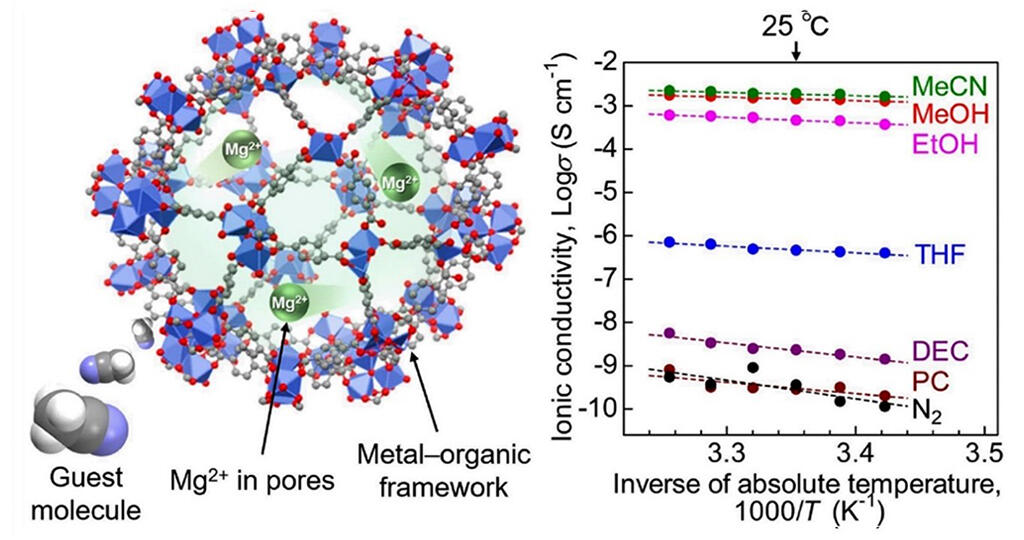
The team first synthesized a MOF know as MIL-101 ⸧ {Mg (TFSI)2}x, in which the Mg salt (TFSI)2 containing magnesium ions, which are ion carriers (ions responsible for conduction), were introduced within the nanopores of a coordination polymer. x is the amount of magnesium ions introduced. The analysis showed that the maximum amount of magnesium ions of the sample with x=1.6 could be encapsulated in the pores.
In addition, in the evaluation of ionic conductivity, they evaluated the ionic conductivity of the sample with x=1.6 containing the maximum amount of magnesium ions because the ionic conductivity was proportional to the concentration of propagating ions and their mobility. Since it is known that the physical properties of coordination polymers, which are porous, change when an external vapor or other substances are adsorbed as guest molecules, they conducted AC impedance measurements in an atmosphere outside the measurement sample that was completely controlled to evaluate ionic conductivity in the presence of various guest molecular vapors. The results show that the synthesized sample exhibits an ionic conductivity of 1.9 × 10-3 S per square centimeter under room temperature (25°C) and acetonitrile vapor. This is the highest reported conductivity for a crystalline solid containing Mg2+ to date.
To further understand the mechanism behind this high conductivity, the research group evaluated the pressure dependence of ionic conductivity and adsorption properties of guest molecules, and also conducted infrared spectroscopic measurements. Under acetonitrile vapor, they found acetonitrile molecules are adsorbed as guest molecules in the pores of coordination polymers and coordinated to magnesium ions, resulting in coordination ion carriers with high mobility, which is a factor in the development of high magnesium ion conductivity.
"I am most interested in the search for a way to create materials that can move any ion in a solid at will, and in establishing the basic science behind this," explains Dr. Sadakiyo. "Moving forward, we would like to create materials that exhibit even higher ionic conductivity and identify the underlying mechanisms. There are many issues that need to be addressed in realizing solid magnesium ion rechargeable batteries, including the electrode materials, and although we are still in the basic research stage, we will continue our research with an eye toward actual battery applications."
■ Coordination polymer: A generic term for solids formed by the self-assembly of metal ions and bridging ligands through coordination bonds.
■ Mobility: An indicator of the mobility of ions.
Journal Information
Publication: Journal of the American Chemical Society
Title: Super Mg2+ Conductivity around 10-3 S cm-1 Observed in a Porous Metal-Organic Framework
DOI: 10.1021/jacs.2c01612
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




