Principal Researcher Satoshi Yasuda of the Research Group for Surface and Interface Science, Advanced Science Research Center, Japan Atomic Energy Agency, and group leader Katsuyuki Fukutani (professor at the Institute of Industrial Science, the University of Tokyo), in collaboration with Hokkaido University and Osaka University, announced that they have demonstrated that hydrogen and deuterium can be separated using a single atom-thick graphene film. The group demonstrated the separation by constructing a solid polymer electrochemical device and quantitatively evaluating the amount of hydrogen and deuterium ions that passed through the membrane. Theoretical calculations further revealed that this separation is due to the quantum tunneling effect. Their findings are expected to lead to the development of low-cost methods of purifying deuterium and were published in the September 1 issue of ACS nano.
Deuterium (D2) is an isotope of hydrogen and is used in a wide range of industries, such as to increase the durability of semiconductor microchips, improve the propagation capacity of optical fibers, and slow metabolisms for deuterated drugs to prolong their efficacy. It is also indispensable in nuclear fusion, which is expected to be a next-generation source of energy.
Currently, the main method of producing deuterium is to separate D2 from a mixture of H2 and D2 gases by cryogenic distillation. While this method can be scaled up, it requires a cryogenic temperature of -250°C and has a low separation ability (H/D resolution).
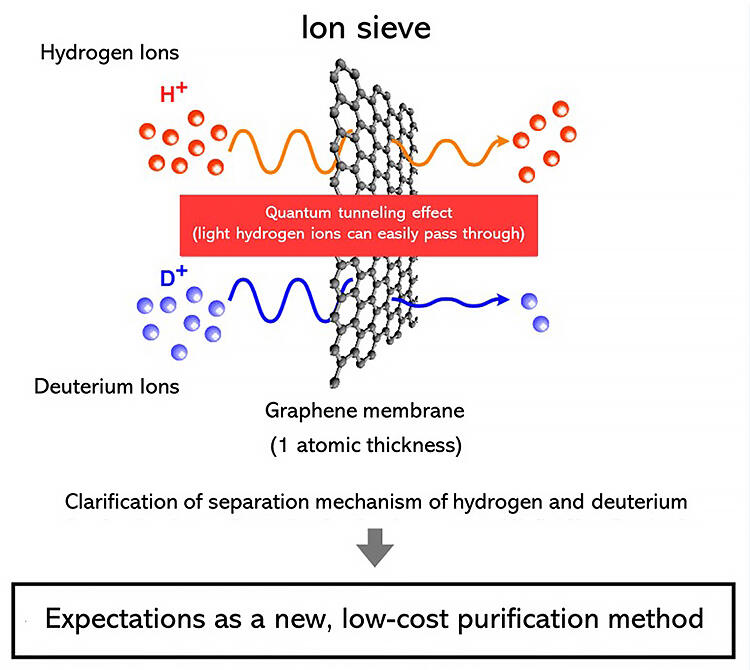
In contrast, graphene films with a thickness of one carbon atom have been recently reported as possibly having the property of allowing the passage of more hydrogen ions than deuterium ions at room temperature. However, although many researchers have conducted follow-up studies, there is no experimental proof of this, and the separation mechanism remains a mystery, leaving researchers divided in their opinions on the subject.
In the group's research, they devised an experimental system using a polymer electrochemical device as a method to verify the H/D resolution of graphene membranes experimentally and with good reproducibly. Polymer type electrochemical devices have the same structure as electric vehicles and household fuel cells in that the solid polymer membrane is sandwiched between the anode and cathode as a single unit. When energy (a voltage) is applied between the two electrodes and a mixture of H2 and D2 gas was supplied to the anode, some H2 and D2 are ionized at the anode to become hydrogen ions and deuterium ions, which flow into the solid electrolyte membrane and react at the cathode to reform hydrogen and deuterium which are then discharged. In their experimental system, a graphene film is fixed between the anode and the polymer film, and the gas emitted from the cathode is examined to evaluate whether the graphene film can function as an "ion sieve" with the ability to separate hydrogen and deuterium.
In the experiment, a graphene film was attached to a solid electrolyte membrane, and a metal film consisting of palladium was overlaid to create an anode. The mixed gas was supplied to the anode and a voltage was applied between the anode and cathode.
As a result, they confirmed with good reproducibility that more H2 was discharged on the cathode side than D2. The ratio of H2 and D2 discharged with and without the graphene membrane differed significantly, and the separation ability was about 10 times higher with graphene, proving that the graphene membrane has an "ion sieve" effect on the H/D separation ability.
In their observations of changes when the voltage was altered, they noted a tendency for the separation ability to decrease as the voltage was increased.
They further studied the mechanism based on this result. Theoretical calculations were conducted using two models, one arising from the specific adsorption of hydrogen and deuterium ions and the other from the quantum tunneling effect, and high consistency was obtained with the model based on the quantum tunneling effect.
The device created in this research operates at about 80°C, and can be handled by modifying products that have already been commercialized, so they anticipate that producing D2 will be cheap and not require cooling.
"To replace the established fabrication method, we believe it is necessary to increase the size and further improve the separation ability of the product," explains Yasuda. "You have to increase the voltage to increase the processing speed, but to do so, you must create a film whose processing capacity does not decrease when the voltage is increased. Therefore, we are currently developing devices with electrode materials that have high resolution at room temperature by using nanomaterials other than graphene films and leveraging the characteristic phenomenon of quantum tunneling."
Journal Information
Publication: ACS nano
Title: Efficient Hydrogen Isotope Separation by Tunneling Effect Using Graphene-Based Heterogeneous Electrocatalysts in Electrochemical Hydrogen Isotope Pumping
DOI: 10.1021/acsnano.2c04655
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




