A research group led by graduate students Akihiro Tanaka and Tomomi Nakano (at the time of research), and Associate Professor Takekazu Kunieda, of the School of Science, the University of Tokyo, in collaboration with a group led by Associate Professor Miho Yanagisawa at the Graduate School of Arts and Sciences and Associate Professor Masaaki Oyama at the Institute of Medical Science, the University of Tokyo, has comprehensively identified a group of proteins that reversibly assemble in response to dehydration-like stress in tardigrades. Furthermore, the group found that a tardigrade-specific cytoplasmic-abundant heat-soluble (CAHS) protein was included in this reservable assembly. The group also discovered that these CAHS proteins form reversible intracellular filaments in response to hyperosmotic stress that enables slower dehydration, improving cells' mechanical strength and hyperosmotic stress tolerance.
Credit: Akihiro Tanaka and Takekazu Kunieda
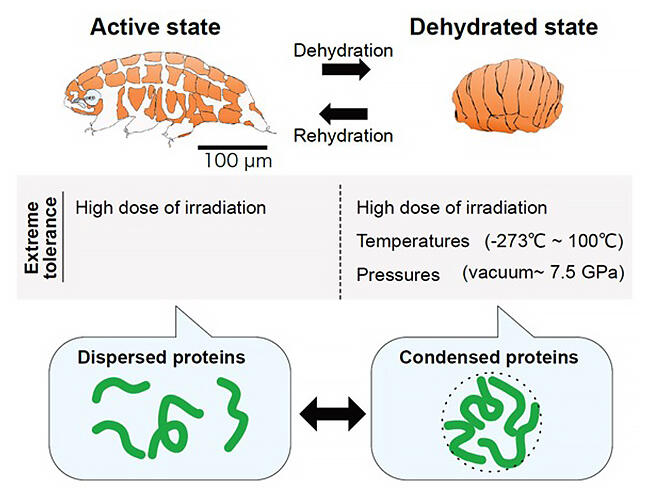
Tardigrades are animals that can withstand almost complete dehydration and various extreme conditions without water. Recently, rapid progress in the molecular biology of tardigrades has led to the discovery of a group of proteins unique to tardigrades as factors involved in their environmental resistance. Still, the mechanism behind this resistance had not been discovered.
The group predicted that some of the nonstructural tolerance proteins in tardigrades might form reversible aggregates in response to stress from dehydration. A new method was established to comprehensively isolate a group of proteins that reversibly assemble in response to dehydration-like stress from tardigrade lysate. As a result, 336 proteins were identified, including a group of CAHS proteins implicated in the tardigrade's tolerance abilities but whose mechanisms were unknown.
Focusing on a portion of the CAHS proteins (CAHS3 and CAHS12) that were present in large quantities, the group fused them with a green fluorescent protein (GFP) to analyze their intracellular behavior and introduced them into human cultured cells. The results showed that CAHS proteins were uniformly dispersed within the cells under non-stress conditions. However, when the cells were exposed to hyperosmotic stress that caused slow dehydration, the CAHS proteins were observed to assemble to form numerous filament-like structures dynamically. The formation of these filament structures was reversible, and after removing the hyperosmotic stress, the CAHS proteins returned to a dispersed intracellular state.
To further investigate the effect of filament formation of the CAHS protein on physical properties, the group prepared cell-sized microdroplets containing the CAHS-GFP protein and measured their elasticity. Under conditions that did not induce filament formation, the droplets were liquid-like with no elasticity. However, significant elasticity was detected under conditions where the filament structures were formed.
Professor Kunieda said, "Tardigrade levels of resistance were not achieved by the proteins identified in this study alone, so we would like to further our comprehensive understanding and application of the tardigrade resistance mechanisms by identifying factors and mechanisms that support this resistance in combination with the discovered mechanisms, as well as through methods of artificially improving these factors and designing new resistant molecules."
Journal Information
Publication: PLOS Biology
Title: Stress-dependent cell stiffening by tardigrade tolerance proteins that reversibly form a filamentous network and gel
DOI: 10.1371/journal.pbio.3001780
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




