A research group consisting of Project Associate Professor Makiko Kashio of the Division of Cell Signaling, National Institute for Physiological Sciences, Professor Satoru Masubuchi of Aichi Medical University and their colleagues has clarified that the temperature at which TRPM2, a body temperature sensor, is activated is connected to the intracellular calcium ion concentration and TRPM2 phosphorylation.
TRPM2 is an ion channel that is activated at high temperatures. As it is expressed in tissues and cells at body temperature, it is thought that its functions at near-body temperature are regulated by some kind of mechanism, but the details of the mechanism that changes TRPM2's temperature sensitivity inside the body were not well understood.
To clarify how TRPM2's functions in tissues at body temperature are regulated the research group focused on and analyzed the role of calcium ions and protein phosphorylation and how they regulate many protein functions. From their results, they learned that when there was a low concentration of calcium ions, TRPM2 did not activate until the temperature rose to around 44℃, but when there was a high calcium ion concentration, it activated at the lower temperature of 34℃.
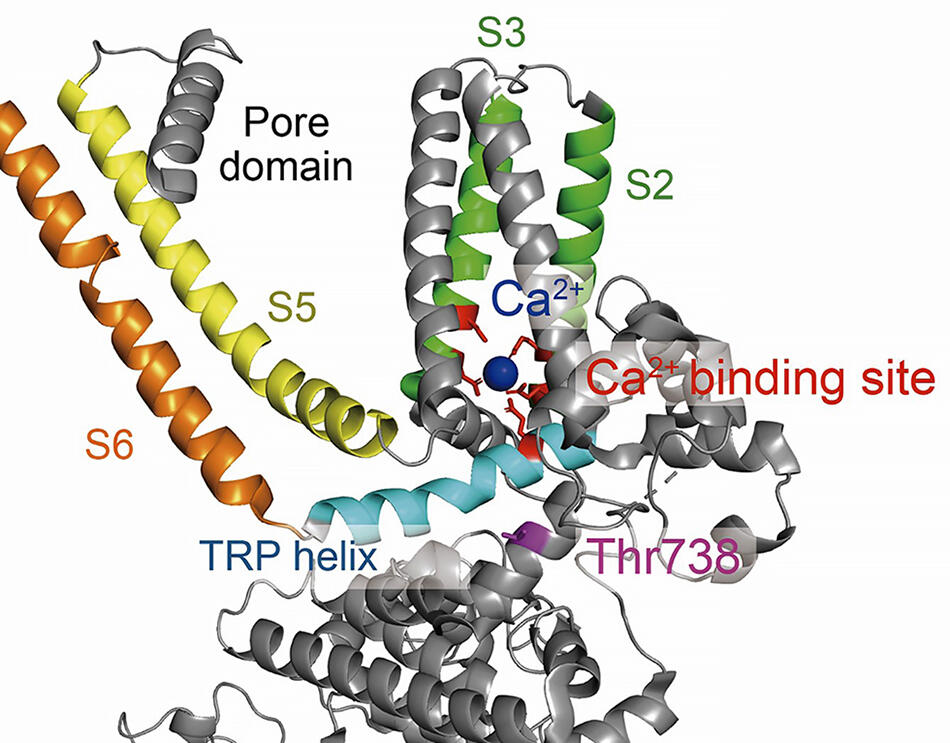
Tertiary structure of the single TRPM2 subunit composed of transmembrane segments (S1-S6), the pore domain and the cytosolic domain. Thr738 residue (magenta) is in the close vicinity of Ca2+ binding site (red) formed by transmembrane S2/S3 segments (green) and TRP helix (cyan).
Provided by National Institute for Physiological Sciences
Moreover, after the group carried out a detailed investigation of the way TRPM2 works, it became clear that TRPM2 undergoes phosphorylation due to PKC (a protein phosphorylation enzyme). Accordingly, they investigated whether the activation temperature changes as a result of TRPM2 phosphorylation, and found that as phosphorylation advances, TRPM2 does not activate unless the temperature is around 42℃ to 44℃, regardless of the calcium ion concentration. This indicated that the phosphorylation of TRPM2 works to negate the effects of intracellular calcium ions.
The group also clarified the amino acid residues connected with regulating the temperatures that activate TRPM2 by analyzing the molecular mechanisms involved. In a variant (Thr738Ala) in which alanine (which does not induce phosphorylation) was substituted for threonine, one of TRPM2's amino acid residues, activation occurred at a similarly low temperature as when there was no phosphorylation, despite treatment with a drug that activated PCK. This demonstrated the possibility that Thr738 is the amino acid responsible for regulating the temperature that induces TRPM2 activation.
Kashio noted, "We clarified the molecular mechanism that determines the temperature (threshold) that activates TRPM2 through the interactions of calcium ions and TRPM2 phosphorylation. We are aware of several TRP channels that activate according to temperature, but do not know the detailed mechanisms behind that temperature-based activation. It is conceivable that this discovery will provide hints to clarify these mechanisms."
Journal Information
Publication: The Journal of Physiology
Title: Protein kinase C-mediated phosphorylation of transient receptor potential melastatin type 2 Thr738 counteracts the effect of cytosolic Ca2+ and elevates the temperature threshold
DOI: 10.1113/JP283350
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




