A research group led by graduate student Akira Nasu, Associate Professor Atsushi Sakuda, President Masahiro Tatsumisago, and Professor Akitoshi Hayashi from the Graduate School of Engineering, Osaka Metropolitan University, in collaboration with the University of Tokyo and Waseda University, has discovered that iron sulfides, which are inexpensive and composed of abundant elements, can be successfully developed into a cathode material (Na2FeS2) using only sodium (Na), iron (Fe), and sulfur (S).
Hayashi et al, Iron Sulfide Na2FeS2 as Positive Electrode Material with High Capacity and Reversibility Derived from Anion-Cation Redox in All-Solid-State Sodium Batteries. CC by 4.0
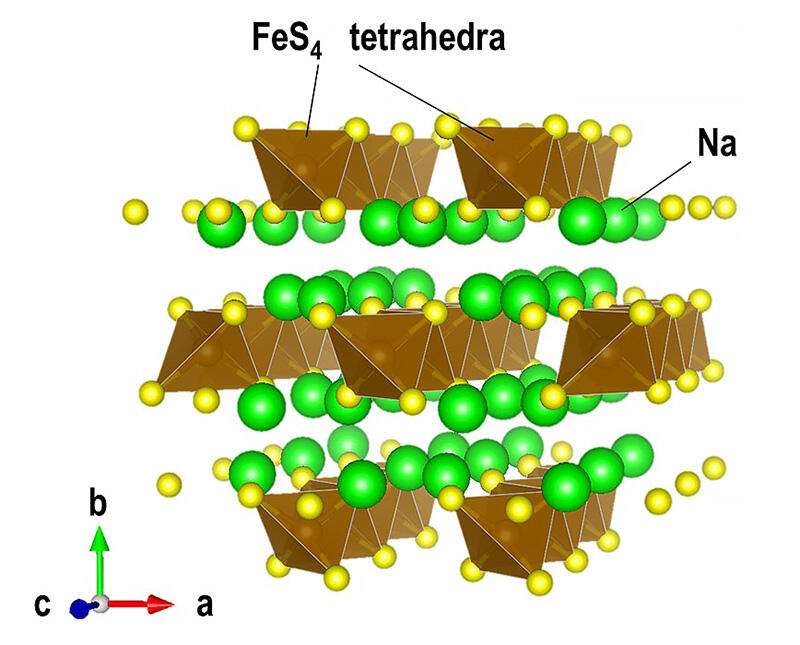
The group has been developing materials consisting of these elements to act as components of all-solid-state sodium rechargeable batteries, treating the use of inexpensive elements as a vital part of its strategy. However, it's not possible to synthesize these materials through simple thermal processing. Instead, a process of firing at high temperatures followed by rapid cooling is required. The group believes further improvements can be made by studying structural changes during charging and discharging.
The all-solid-state sodium battery prototype using Na2FeS2 as the cathode active material showed a high capacity of about 320 mAh/g per weight of Na2FeS2, which was equivalent to the theoretical capacity. The group also found that the resulting battery can be reversibly charged and discharged for more than 300 cycles. It determined that the battery's long life is due to the unique crystal structure of Na2FeS2. While most general high-capacity metal sulfide electrode materials exhibit conversion-type charge-discharge reactions, the Na2FeS2 material mainly exhibits an insertion-type charge-discharge reaction that retains its structure. This trait is thought to be the reason for its high reversibility.
Associate Professor Sakuda said, "This material shows high performance in terms of ease of mass production, the abundant resources it's made from, its longevity, and its energy density. To enable practical use, we need to work to enable faster charging and discharging. In the future, we would like to add a small amount of other elements to the iron portion of Na2FeS2, improving the battery to enable these characteristics."
■ Conversion type: Charge-discharge reactions involving large chemical bonds and structural changes are called conversion-type reactions.
■ Insertion type: Charge-discharge reactions in which insertion and desorption of mobile ions (sodium ions) occur while the structure is partially retained are called insertion-type reactions.
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




