Keratin-associated proteins are promising therapeutic targets expected to help develop novel therapeutic strategies.
A research group led by Professor Tetsuro Watabe, Assistant Professor Katarzyna A. Podyma-Inoue, and collaborative researcher Kazuki Takahashi, of the Department of Biochemistry, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University (TMDU), in collaboration with the University of Tokyo, Osaka University and Wakayama Medical University, has discovered a new mechanism by which cell cycle-arrested oral cancer cells gain motility and metastasize. This is expected to clarify the pathologies of various cancers and help develop new treatment methods. This research was published in the online edition of Cell Reports on September 27.
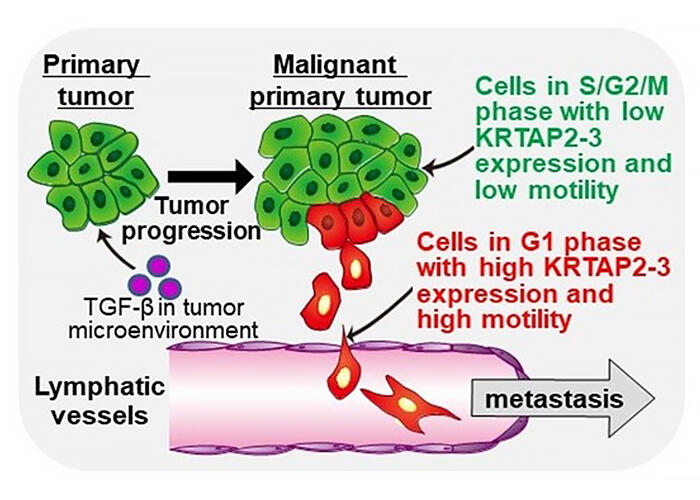
Takahashi et al. show that TGF-β generates a population of cancer cells under cell cycle arrest in G1 phase with high motility by inducing unique Slug/HMGA2-independent epithelial-mesenchymal transition (EMT). This leads to tumor progression and poor prognosis for patients with head and neck cancer.
Provided by TMDU
The study was conducted in collaboration with other research groups led by Distinguished University Professor Kohei Miyazono of the Graduate School of Medicine, the University of Tokyo, Assistant Professor Toshihiro Uchihashi of the Graduate School of Dentistry, Osaka University, Professor Mariko Okada of the Institute for Protein Research, Osaka University, Professor Shinichi Hashimoto of the School of Medicine, Wakayama Medical University, and Professor Masahiko Miura of the Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University (TMDU).
The research was supported by the Japan Agency for Medical Research and Development (AMED) Project for Promotion of Cancer Research and Therapeutic Evolution (P-PROMOTE), the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grants-in-Aid for Scientific Research, and other sources.
The group found that oral cancer cells that entered into G1 cell cycle arrest in response to transforming growth factor-β (TGF-β), exhibit high motility and metastatic ability. TGF-β is a cytokine abundant in tumor microenvironment that regulates various cellular activities. TGF-β is known to inhibit proliferation of epithelial cancer cells and to play an important role in various pathological conditions such as carcinogenesis and cancer invasion/metastasis by regulating cell differentiation and motility.
In this study, the group discovered a novel mechanism by which TGF-β induces epithelial-mesenchymal transition (EMT) in oral cancer cells via upregulation of the expression of keratin-associated protein 2-3 (KRTAP2-3), a poor prognostic factor for patients with head and neck cancer. The cell cycle, the process through which cells pass during division, consists of the G0 phase (division arrest), G1 phase (preparation for DNA synthesis), S phase (DNA synthesis) G2 phase (preparation for division), and M phase (division). TGF-βelicits dual effect on epithelial cancer cells. It exhibits "cancer-promoting" effect via induction of EMT and "cancer-suppressing" effect by arresting the cell cycle in the G1 phase (the phase during which cells prepare for DNA synthesis), but the relationship between these two opposing effects has yet to be clarified.
The group used oral cancer cells carrying the Fucci reporter system, which allows for monitoring the cell cycle progression at a signal cell level and enables separation of proliferating cells in the S/G2/M phases from cells in the G1 phase by FACS. When they performed migration assays, they found that the cells in G1 phase under cell cycle arrest, induced by TGF-β, exhibit high motility and mesenchymal characteristics. Single-cell RNA sequencing analysis revealed that these cells under cell cycle arrest in the G1 phase arose via a unique TGF-β-induced EMT pathway which is distinct from the previously reported EMT pathway mediated by canonical EMT-related HMGA2 transcription.
To further shed light on the mechanism of this phenomenon, the group identified KRTAP2-3 as a factor whose expression is upregulated in cells in the G1 phase by TGF-β. They also found that KRTAP2-3 is correlated with tumor progression in patients with head and neck cancer and identified it as a regulator of the dual action of TGF-β, as it inhibits proliferation and enhances motility when expressed in cancer cells.
In an orthotopic transplantation model in mice, oral cancer cells lacking the KRTAP2-3 gene showed reduced lymph node metastasis. In addition, cells lacking KRTAP2-3 underwent mesenchymal-epithelial transition (MET), a process reversal of EMT, indicating that KRTAP2-3 is a novel factor that suppresses growth of oral cancer cells while enhancing their motility and metastatic potential via EMT induction, and is a promising therapeutic target.
The present findings, that TGF-β oral cells with suppressed proliferative ability exhibit increased motility and high metastatic potential, changed the current paradigm in cancer biology. The present data also suggest that by inhibition of KRTAP2-3 expression and function we can suppress the metastasis of oral cancer. KRTAP2-3 expression in normal tissues is restricted to hair shafts but is upregulated in cancer cells such as head and neck cancer and gastric cancer, thus making it as a potential new therapeutic target for various types of cancer.
Journal Information
Publication: Cell Reports
Title: TGF-β generates a population of cancer cells residing in G1 phase with high motility and metastatic potential via KRTAP2-3
DOI: 10.1016/j.celrep.2022.111411
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




