A research group consisting of Project Professor Yukiko Sasazawa of the Research Institute for Diseases of Old Age, Graduate School of Medicine, Juntendo University, Senior Associate Professor Shinji Saiki and Professor Nobutaka Hattori of the Department of Neurology, Graduate School of Medicine, Juntendo University and their colleagues has announced that they have discovered an increase in acrolein, a toxic aldehyde, in the blood of Parkinson's patients, and clarified that autophagy works as a defense response to this. Lysosomes exposed to acrolein move to the microtubule-organizing center (MTOC). The group has clarified a new molecule associated with this movement. It is hoped that these outcomes will lead to the development of new therapeutic drugs for Parkinson's disease. Their outcomes were published in the October 11 edition of the international science journal The EMBO Journal.
Oxidative stress-induced phosphorylation of JIP4 regulates lysosomal positioning in coordination with TRPML1 and ALG2. CC by 4.0
Parkinson's disease is the second most common progressive neurodegenerative disease in Japan and is designated as an intractable disease. There is no complete cure for Parkinson's disease, and there are many uncertainties regarding the pathogenic mechanism, but in recent years a connection with autophagic failure has been reported. Autophagy is a system vital to the homeostasis of cells, which breaks down unneeded cell components by enveloping them in a double membrane called an autophagosome and sending them to lysosomes.
In this study, the research group discovered a striking increase in acrolein in the blood of Parkinson's patients by comparing the blood components of 94 Parkinson's patients and 22 healthy people.
Next, they treated cultured cells with acrolein to clarify what was happening in cells with increased acrolein. When they did so, the lysosomes, which had been scattered in the cells before treatment, accumulated around the MTOC. The group learned that this is where autophagy is activated.
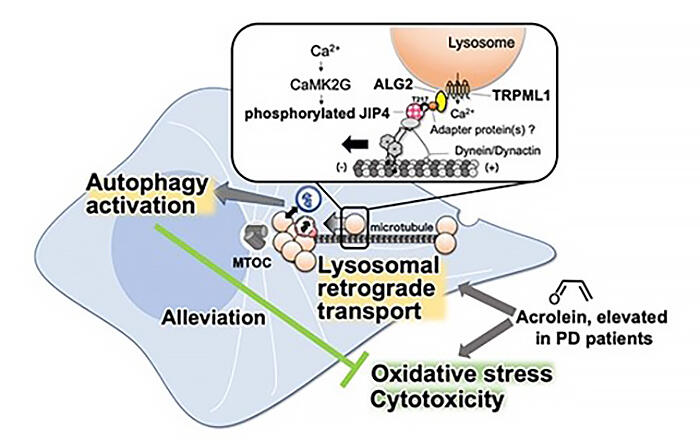
In past studies, lysosomes were seen to travel via the activity of motor proteins on microtubule rails inside the cells. The lysosomes were reported to attach to motor proteins through adaptor proteins. It was also understood that combinations of these led to the existence of multiple varieties.
When the group explored molecules connected with the change in lysosome distribution due to acrolein, they clarified that this is associated with ALG2 and TRPML1, as well as JIP4. They also learned that TMEM55B, which was thought to have a part in this, is not actually involved.
The complexes formed by ALG2 and TRPML1 interacted with JIP4 as a result of acrolein treatment. It became clear that when this happens JIP4 is phosphorylated (T217), and that the phosphorylating enzyme CaMK2G, activated by calcium, is responsible for this. When CaMK2G activity is inhibited, both the changed distribution of lysosomes due to acrolein and autophagy are also inhibited.
From this information, the group discovered that acrolein treatment causes the lysosomes to release calcium, CaMK2G is activated and JIP4 is phosphorylated. It became clear that the phosphorylated JIP4 forms complexes with ALG2 and TRPML1, creating a series of mechanisms associated with the transport of lysosomes.
Acrolein induces oxidative stress due to things such as aging. When cells were treated with hydrogen peroxide, which demonstrates a similar effect, the group observed lysosomes accumulating around the MTOC, and similar JIP4 phosphorylation occurred. Based on this, the group believe that the activation of autophagy due to phosphorylated JIP4 is a response to oxidative stress.
They also investigated what this phenomenon causes in cells. Comparing the survival rates of cells treated with acrolein - both cells that indirectly inhibited the expression of JIP4 and regular cells - showed that the JIP4 inhibited cells had lower survival rates than regular cells.
This sheds light on the idea that the lysosome gathering and autophagy activation for which JIP4 is responsible is a system that protects cells from oxidative stress. Autophagy can also be induced by starvation; the system clarified in this study is seen as a new defensive system induced due to oxidative stress.
Sasazawa commented, "We believe that if there was a drug that could activate the mechanism we have now discovered, it could lead to drug discovery for Parkinson's disease in the future; we are currently exploring this avenue of research."
Journal Information
Publication: The EMBO Journal
Title: Oxidative stress-induced phosphorylation of JIP4 regulates lysosomal positioning in coordination with TRPML1 and ALG2
DOI: 10.15252/embj.2022111476
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




