A research group led by Honorary Professor Hideo Hosono, Specially Appointed Assistant Professor Yangfan Lu (currently Associate Professor at Chongqing University), and Associate Professor Masaaki Kitano of the Materials Research Center for Element Strategy, Tokyo Institute of Technology, has created a new noble metal-free catalyst with the high water resistance required for synthesis of ammonia with a low carbon footprint in the presence of moisture.
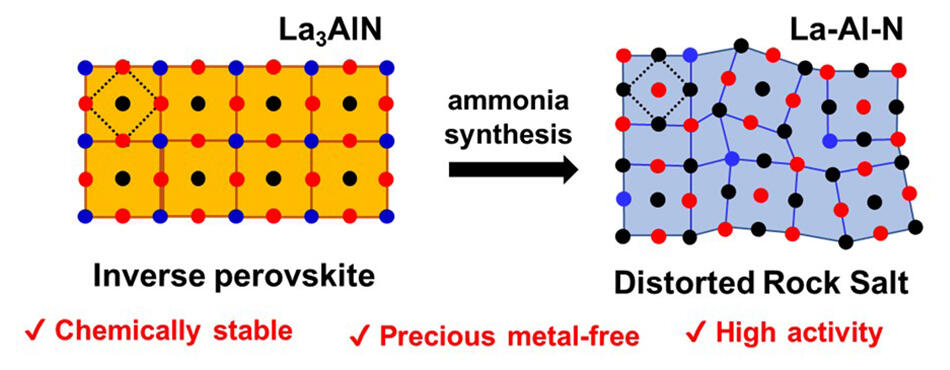
Left: starting structure (chemically stable but not active), Right: transformed structure (stable and active).
Provided by Tokyo Institute of Technology
Ammonia is extremely important for today's society and is produced in large quantities using the Haber-Bosch process, a method of synthesizing ammonia from nitrogen and hydrogen under high temperatures and high pressure. However, the process of making hydrogen from methane requires a large amount of energy that results in the production of large amounts of carbon dioxide. For this reason, research on ammonia with a low carbon footprint, which uses renewable energy to electrolyze water to produce hydrogen that is then used to synthesize ammonia under milder conditions, has been gaining momentum worldwide. Rare metals such as ruthenium have been the main catalysts studied to achieve this.
In 2020, the research group carried out strong dissociation of nitrogen molecules using nitrogen defects on the surface of nitrides based on the idea of the strong electron donation ability and interactions between supports and active metals, and reported that a nickel-supported lanthanum nitride catalyst, which had not received much attention due to its low activity, was comparable to a ruthenium catalyst. However, the disadvantage of this catalyst was that it was sensitive to moisture and its activity was greatly reduced when exposed to the air. This instability with respect to water is common to catalysts with high activity under mild conditions and is a challenge that must be overcome for practical application.
Instead of lanthanum nitride, the new catalyst is made of La3AlN, which is stable in moisture, with nickel or cobalt supporting it. La3AlN is transformed into Al-doped lanthanum nitride (La-Al-N) during the ammonia synthesis process, greatly improving the chemical stability of the catalyst surface due to the stabilizing effect of the La-Al bond. The La-Al-N catalyst does not lose its catalytic activity even when exposed to oxygen or moisture and combines specific catalytic functionality originating from nitrogen vacancies with high resistance to oxygen and water.
"This achievement was obtained based on the idea of using a stable starting material to convert a highly active but moisture-sensitive catalyst into a highly active and stable substance during the reaction," explained Hosono. "Moving forward, we need to increase the specific surface area and optimize the aluminum concentration in order to achieve even higher activity."
Journal Information
Publication: Angewandte Chemie
Title: Approach to Chemically Durable Nickel and Cobalt Lanthanum-Nitride-Based Catalysts for Ammonia Synthesis
DOI:10.1002/anie.202211759
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




