A research group led by Associate Professor Eiji Yuba of the Graduate School of Engineering, Osaka Metropolitan University, has successfully developed a material for drug delivery systems (DDS) that can activate a powerful cellular immunity by incorporating cationic lipids into liposomes, microcapsules that contain cancer antigens, and modifying their surface with pH-responsive polysaccharides. "Incorporating cationic lipids increased the uptake efficiency of liposomes into dendritic cells five-fold, and the amount of IL12 produced increased 100-fold compared to conventional technologies," explained Yuba. "Moving forward, we would like to contribute to research on the practical application of antigen carriers to expand the use of cancer immunotherapy and to the development of next-generation mRNA vaccines." The group's research was published online in the Journal of Controlled Release.
Provided by Eiji Yuba, Osaka Metropolitan University
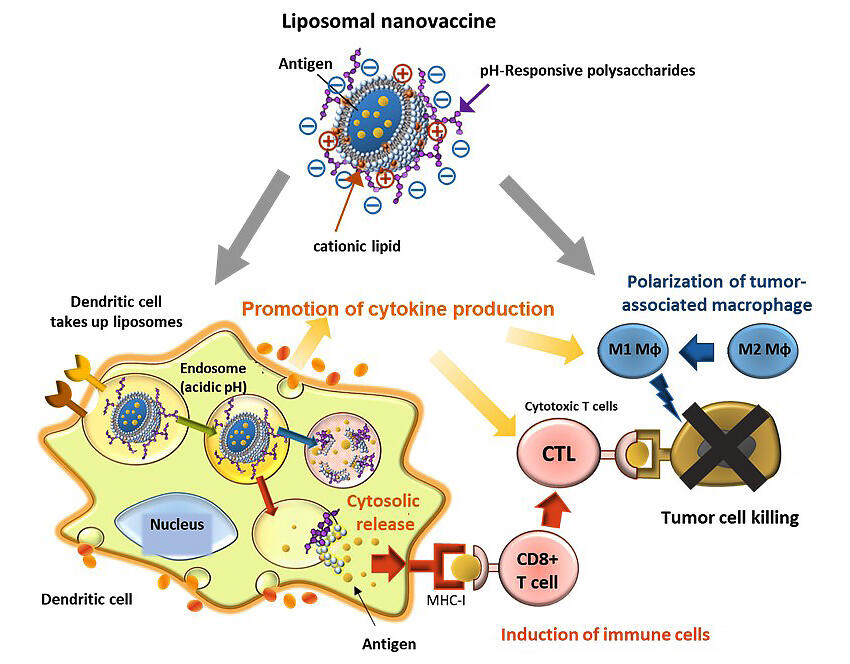
Antigens must be transported to and activated by antigen-presenting cells and released from intracellular acidic vesicles (endosomes) in order to induce cellular immunity. The research group has focused on nanocapsules (liposomes) made of phospholipids as antigen carriers. To date, the group has succeeded in simultaneously achieving the intracellular release of antigens and activation of antigen-presenting cells, thereby inducing cell-mediated immunity, by using a pH-responsive polysaccharide that has the function of destroying lipid membranes in response to a weakly acidic environment.
In this research, they incorporated positively charged cationic lipids into pH-responsive polysaccharide-modified liposomes to induce cellular immunity even more potently than before. Cationic lipids themselves not only have the effect of activating immunity, but also effectively immobilize negatively charged pH-responsive polysaccharides on liposomes through electrostatic interactions by positively charging the liposome membrane surface.
Increasing the percentage of incorporated cationic lipids to 13% increased the amount of pH-responsive polysaccharide immobilized on liposomes by a factor of about three, resulting in a more negatively charged liposome surface. The pH-responsive polysaccharide-modified liposomes with cationic lipids were taken up by dendritic cells about five times more efficiently than those without cationic lipids.
Furthermore, when they mixed a mouse-derived dendritic cell line with pH-responsive polysaccharide-modified liposomes with cationic lipids and studied the number of liposomes taken up by dendritic cells after four hours, liposomes loaded with 13% cationic lipids were taken up over 20 times more than unmodified liposomes. They found that dendritic cells into which cationic lipids were introduced secreted a larger number of cytokines than those without cationic lipids, and that dendritic cells were strongly activated. In particular, when they used β1,3-glucan as the main chain of the pH-responsive polysaccharide, incorporating cationic lipids increased the production of IL12 about 100-fold, indicating that the combination of polysaccharide with a specific structure and cationic lipids was effective in activating antigen-presenting cells.
Next, they administered pH-responsive polysaccharide-modified liposomes containing cancer antigens and cationic lipids near the cancer tissue of mice that had developed cancer, which inhibited the growth of cancer. There was a marked increase in the number of cells that recognized the antigen and produced IFNγ in the spleens of the mice, indicating that antigen-specific cellular immunity was induced. Furthermore, when the group examined the percentage of immune cells in the cancer tissues, they saw a trend toward an increased percentage of M1 macrophages involved in immune activation compared to M2 macrophages.
The powerful ability of liposomes to activate immune cells is thought to have reduced the number of immunosuppressive macrophages in the tumor and to have attacked the cancer via cellular immunity induced by the intracellular transport of antigens. Furthermore, even though the amount of antigen administered was one-tenth that of the conventional dose, it induced such strong cancer immunity that the cancer disappeared in some mice.
"These results indicate that the combination of cationic lipids and pH-responsive polysaccharides with immune cell activating capacity is effective in creating potent liposomal cancer vaccines," noted Yuba. "In the future, we would like to conduct basic research to develop antibody carriers that can activate cancer immunity more effectively by optimizing the pH-responsive successful molecular structure."
Journal Information
Publication: Journal of Controlled Release
Title: Cationic lipid potentiated the adjuvanticity of polysaccharide derivative-modified liposome vaccines
DOI: 10.1016/j.jconrel.2022.10.016
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




