The research group led by Assistant Professor Kohei Tsujimoto, Lecturer Hyota Takamatsu, and Professor Atsushi Kumanogoh of the Graduate School of Medicine at Osaka University, in collaboration with Kyoto University and the Telethon Institute of Genetics and Medicine, has announced that they have brought to light a mechanism that regulates lysosomal inflammation that is associated with pathological conditions such as gout. The research group found that the Ragulator complex (a protein complex) on lysosomes regulates the inflammatory response induced by the NLRP3 inflammasome, and the action of the Ragulator complex is inhibited by a synthetic form of vitamin E. They also confirmed that injections of this molecule reduced symptoms in gout model mice. These results are expected to lead to the development of new drugs for gout, arteriosclerosis, Alzheimer's disease, and other diseases in which the NLRP3 inflammasome is involved. The results were published in the November 29 issue of the international scientific journal The EMBO Journal.
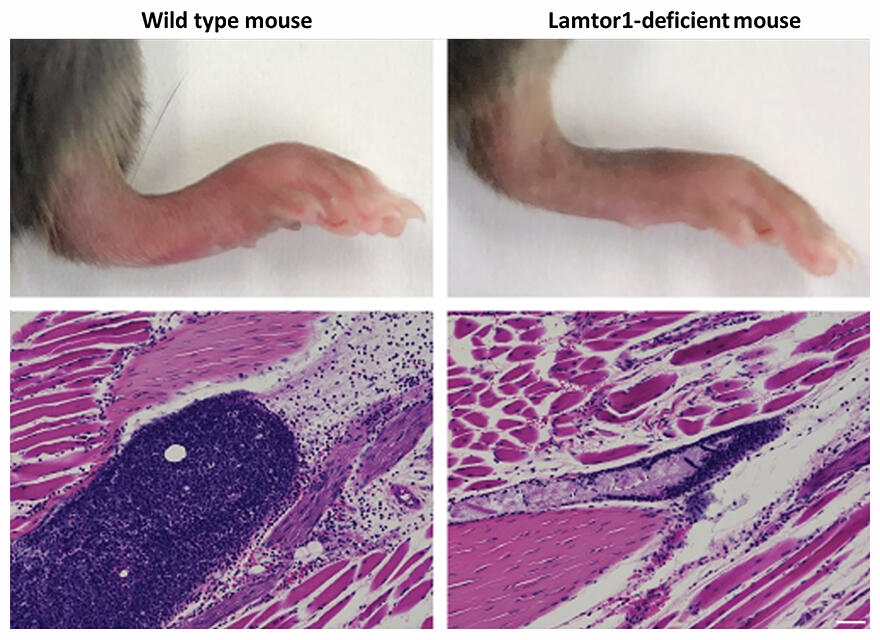
(Above): reduced swelling and redness
(Below): reduction in number of inflammatory cells
Provided by Osaka University
Living organisms have a system for detecting and eliminating invading pathogens and foreign substances, and the inflammatory response by the NLRP3 inflammasome (protein complex) is a typical example of this. NLRP3 inflammasomes are formed by recognizing not only components of microbial origin, but also double-stranded DNA that is released from injured cells, uricemia (the cause of gout), and asbestos, resulting in an inflammatory response, but this regulatory mechanism is complex and is not well understood.
Lysosomes are known to be cell organelles that perform intracellular digestion, but recently it has become clear that lysosomes also have various other functions and act as intracellular command centers. To date, the research group has been focusing on lysosomes. To elucidate the molecular basis of lysosomes, they have focused their research on the Ragulator complex that exists on lysosomes. The Ragulator complex is a protein complex consisting of Lamtor1-5 subunits, and the research group has shown that Lamtor1 regulates inflammation and cell movement.
The research group began their work with the hypothesis that the Ragulator complex regulates the formation and activity of the NLRP3 inflammasome. To investigate the functions of the Ragulator complex, they generated mice that were deficient in Lamtor1 (which prevented the Ragulator complex from being formed) in monocytes and macrophages, and artificially induced gout in them. The same treatment caused gout-like inflammation and cellular infiltration in wild mice, but these symptoms were significantly reduced in mice lacking the Ragulator complex. Similarly, it was also confirmed that the absence of the Ragulator complex in mouse bone marrow-derived macrophages and human monocyte-like cell lines did not activate the NLRP3 inflammasome.
Next, the researchers generated a mutant in which the Ragulator complex was absent only on the lysosomes and found that the NLRP3 inflammasome was not activated in this mutant. This indicated that protein-protein interactions on the lysosome are important for the activation of the NLRP3 inflammasome.
In order to clarify this interaction, they performed a variety of analysis, including mass spectrometry of lysosomes, which showed that HDAC6 and NLRP3 bind to the Ragulator complex and that NLRP3 cannot bind to the Ragulator complex in the absence of HDAC6.
Because it was reported that NLRP3 and HDAC6 bind, this clarified that the binding between the Ragulator complex and NLRP3 is regulated by the binding between the Ragulator complex and HDAC6.
Using a library of natural compounds, the research group searched for compounds that inhibit the activation of the NLRP3 inflammasome through this interaction. As a result of their search, they identified synthetic vitamin E and then actually confirmed its binding inhibition effect through experiments.
Synthetic vitamin E is already used in the treatment of dyslipidemia and peripheral circulatory failure, and its antioxidant effect is thought to be central to its effectiveness. To investigate whether this inhibition is due to the antioxidant effect, they compared the inhibitory effect with natural vitamin E, which has a higher antioxidant effect than synthetic vitamin E. In doing so, it was found that the inhibition effect of natural vitamin E was lower than that of synthetic vitamin E, indicating that this inhibition effect was due to a new mechanism.
The research group also confirmed that injecting synthetic vitamin E into inflammatory sites for gout and peritonitis in mouse models significantly reduced inflammation.
Journal Information
Publication: The EMBO Journal
Title: The lysosomal Ragulator complex activates NLRP3 inflammasome in vivo via HDAC6
DOI: 10.15252/embj.2022111389
This article has been translated by JST with permission from The Science News Ltd.(https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




