Human pluripotent stem cells (hPSCs) (ES cells/iPS cells) do not actually exist in the body, so they have mutations unique to test-tube production. A representative example is the mutation of the specific sex chromosome in female PSCs, the X chromosome, known as the irreversible erosion of X chromosome inactivation (XCI).
A research group consisting of Lecturer Atsushi Fukuda of the School of Medicine, Tokai University and his colleagues has succeeded in restoring the irreversibly lost XCI in female PSCs through joint research with the Center for Regenerative Medicine, National Center for Child Health and Development. They also confirmed neuronal differentiation in iPS cells from patients with restored XCI, and demonstrated the possibility of accurate pathogenic modeling research. Their outcomes were published in the online version of Cell Reports Methods.
Provided by Tokai University
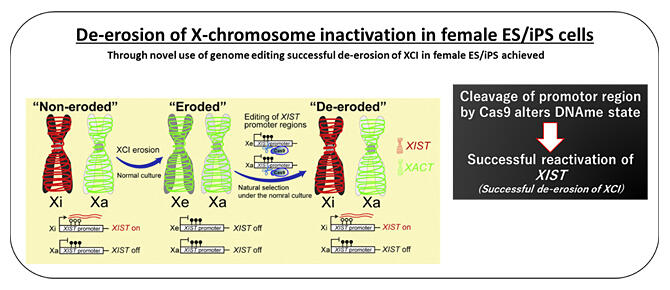
X chromosome inactivation (XCI) is controlled by XIST. In the past, the research group clarified that the suppression of XIST expression is induced through DNA methylation in the promoter regions that control XIST expression.
This study explored the possibility of the demethylation of mutated DNA methylation in XIST regions through DNA editing. From the outcomes, the group learned that DNA editing in the XIST promoter regions induces DNA demethylation and reactivates XIST. More specifically, the group clarified that it is possible to use DNA editing by Cas9 nuclease to alter the state of DNA methylation in edited regions and reactivate genes. They also made it clear that XCI is ultimately recovered in female PSCs in which XIST has been reactivated. This phenomenon was confirmed in all tested female ES/iPS cells, and a high level of reproducibility was proven (a patent application has been made).
In addition, the group clarified that normal neuronal differentiation took place in cells in which XCI has been recovered, in contrast to iPS cells derived from Rett syndrome patients whose XCI had eroded, in which neuronal development is poor. With these outcomes, neuronal differentiation in iPS cells derived from Rett syndrome patients has made it possible to analyze the effect of MECP2, the main cause of Rett syndrome, for the first time by normalizing the state of XCI, leading to the development of core technology for pathological modeling using disease-afflicted iPS cells with normal XCI.
It is hoped that these outcomes will dramatically widen the scope of female PSC application, as well as leading to the clarification of pathological mechanisms unique to women.
Journal Information
Publication: Cell Reports Methods
Title: De-erosion of X-chromosome dosage compensation by editing of XIST regulatory regions restores differentiation potential in hPSCs.
DOI: 10.1016/j.crmeth.2022.100352
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




