A research group led by Senior Researcher Yuki Kohno, Mitsuhiro Kanakubo, and Group Leader Takashi Makino of the Research Institute for Chemical Process Technology at the National Institute of Advanced Industrial Science and Technology (AIST), in collaboration with Daicel Corporation (Daicel), has successfully developed a membrane for the highly selective separation and recovery of dilute CO2, such as CO2 in the atmosphere.
Provided by AIST
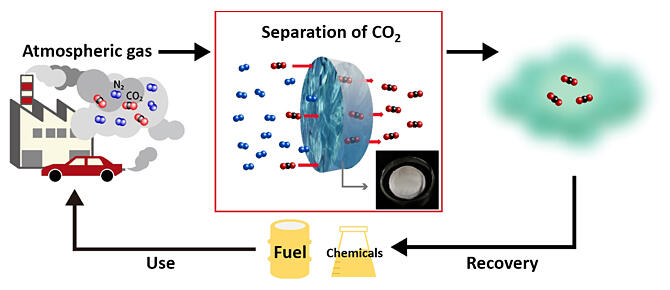
Carbon recycling technology, which separates and recovers CO2 emitted into the atmosphere and reuses it as a raw material for synthesizing various carbon compounds as carbon resources, is attracting attention to help achieve carbon neutrality by 2050. In recent years, technologies using chemical absorption and chemical adsorption have been demonstrated in Europe and the United States, but their disadvantage is that they consume a large amount of heat to recover the CO2 absorbed or adsorbed in the separation material.
The research group focused on the membrane separation method, a CO2 separation technology that, in principle, does not require thermal energy. CO2 separation and recovery using chemically reactive membrane materials requires rapid progression of CO2 absorption into and desorption from the membrane. As such, the researchers achieved faster absorption and desorption by mixing two kinds of ionic liquids with distinct roles. They developed a mixture of ionic liquids that chemically react with CO2 and ionic liquids that solvate with compounds formed by the chemical reaction (ionic liquid mixture). They produced an ionic liquid membrane by impregnating a porous material with this ionic liquid mixture. A model gas mixture of CO2 and N2 (CO2 concentration: 0.04%) was supplied upstream of the membrane, and He was supplied downstream as the sweep gas.
The CO2/N2 selectivity reached about 200 times higher than that of conventional membranes in tests using 0.04% CO2, which is comparable to CO2 in the air. Furthermore, by developing an ionic liquid with an optimized molecular structure of anions, they succeeded in developing an ionic liquid membrane with a CO2/N2 selectivity of more than 10,000 times, and eventually reduced CO2 to about 70%. This is the highest class of performance as a material for separation membranes for DAC (separation and recovery technology to reduce CO2), and its CO2/N2 selectivity is equivalent to about 500 times that of conventional polymer membranes that show equivalent CO2 permeability.
"In the future, we will develop manufacturing and modularization technologies for ionic liquid membranes to apply DAC technology using mixed ionic liquid membranes," explained Makino, leader of the research group. "To achieve carbon neutrality, it is also essential to separate and recover CO2 not only from the atmosphere, but also from a variety of CO2 sources. The concept of mixed ionic liquid membranes is not limited to atmospheric CO2 separation and recovery. We would like to develop the separation membranes for various sources of CO2 emissions."
Journal Information
Publication: ACS Omega
Title: Ionic Liquid Mixtures for Direct Air Capture: High CO2 Permeation Driven by Superior CO2 Absorption with Lower Absolute Enthalpy
DOI: 10.1021/acsomega.2c04756
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




