A research group consisting of Professor Naoki Watanabe of the Institute of Low Temperature Science, Hokkaido University, and his colleagues has worked with Senior Research Scientist Yoichi Nakai of the RIKEN Nishina Center for Accelerator-Based Science and succeeded in using a newly developed technique to investigate the activation temperature of OH radicals on an extremely low-temperature ice dust surface.
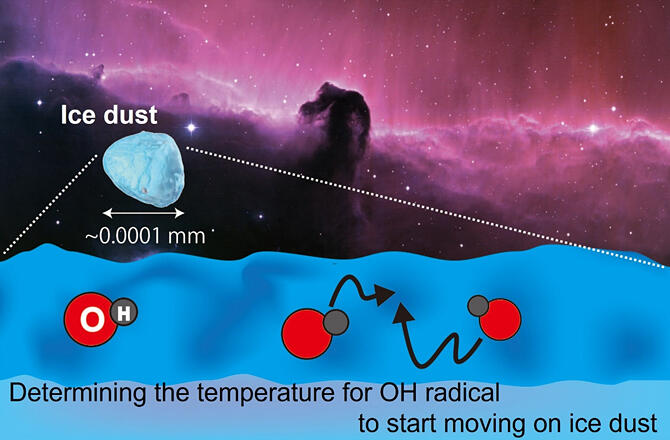
An OH radical is a water molecule (H2O) minus a hydrogen atom (H), and one of its properties is that it is highly reactive. There are large numbers of OH radicals on the surface of extremely low temperature interstellar ice dust particles, and it is thought that they play a major role in the formation of various molecules, including organic molecules. How were molecules created in extremely low-temperature space (−237℃), long before stars were born? How complex was their evolution? The key to these questions lies in the unique chemical reactions that occur on the surface of extremely low-temperature fine interstellar ice dust (around 0.0001 mm). It is thought that the reactions of the highly reactive OH radicals on the ice surface have a particularly important role in this. However, an uncommonly high-sensitive and non-destructive detection method was needed to recreate interstellar ice particles in a laboratory and investigate the behavior of OH radicals on the ice surface.
Provided by Hokkaido University
The research group reproduced extremely low-temperature fine interstellar ice particles within vacuum experiment equipment developed by the Institute of Low Temperature Science, and generated OH radicals on the ice surface by using ultraviolet light irradiation to separate some water molecules (H2O→OH+H). The group observed the OH radicals on the ice surface by pulling them from the ice surface via laser beams and using another laser to analyze and detect the OH radicals released. When the OH radicals reached their activation temperature on the ice surface, they reacted with each other (OH+OH→H2O2), so the number of OH radicals decreased. To investigate the relationship between this temperature and the number of OH radicals, the group measured the energy needed for the OH radicals on the ice surface to start moving.
From the energy needed for the OH radicals to start moving, which they determined from experiments, the group calculated the speed at which the OH radicals move around on the ice surface under various temperature conditions. It is necessary to think about chemical evolution on fine ice particles in a timescale of 100,000 years, and so the group estimated temperatures that allowed these to move all over the surface of interstellar ice dust (diameter: around 0.0001 mm) during that 100,000 years.
Consequently, they learned that once the temperature exceeds around −237℃ on the surface of the fine interstellar ice particles that exist in the extremely low-temperature space environment, OH radical movement becomes active and molecular evolution is facilitated. As the formation of stars in interstellar molecular clouds advances, the temperature of the environment gradually increases. At extremely low temperatures of around −263℃, the OH radicals that have accumulated on the ice surface begin moving as the temperature rises, causing active molecular evolution. Using predetermined temperatures to run simulations of chemical evolution allowed the group to gain a more accurate understanding of the molecular evolution process associated with OH radicals.
Watanabe commented, "It took a long time to develop a method that made use of two types of lasers to release OH from the ice surface and analyze it. In the future, we want to use this technique we have developed to investigate the behavior of radicals such as CH3 and HCO, which are the basic materials for complex organic molecules connected to the origins of life, on the ice surface."
Provided by Hokkaido University
Journal Information
Publication: The Astrophysical Journal Letters
Title: Direct Determination of the Activation Energy for Diffusion of OH Radicals on Water Ice
DOI: 10.3847/2041-8213/ac9d30
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




