A research group consisting of Professor Motohiro Nishida, Assistant Professor Yuri Kato and their colleagues of the Graduate School of Pharmaceutical Sciences, Kyushu University, worked with researchers from the National Institute for Physiological Sciences/Exploratory Research Center on Life and Living Systems, Shinshu University, Kyoto University, and other institutions, using transgenic mice to clarify that in blood vessels after hindlimb ischemia, vascular endothelium-derived relaxing factor (EDRF) suppresses channel activity through the phosphorylation of TRPC6 proteins in vascular smooth muscle cells, and consequently facilitates vascular maturation. They also succeeded in obtaining the compound 1-BP (1-benzyl-1-(11-hydroxyundecyl)piperidin-1-ium chloride), which facilitates blood-flow recovery most strongly after hindlimb ischemia, from compounds with TRPC6 channel inhibitory activity. This result was achieved through collaboration with Distinguished Professor Takashi Ohshima and his colleagues from Green Pharmaceutical Chemistry, Graduate School of Pharmaceutical Sciences, Kyushu University.
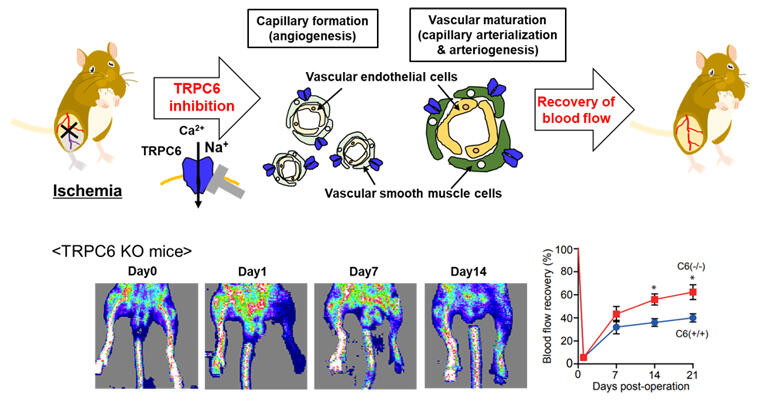
Suppression of TRPC6 channel activity promotes capillary formation and vascular maturation regardless of endothelial functions and facilitates blood flow recovery after ischemia.
Provided by Kyushu University
Peripheral circulatory damage, in which blood gradually stops flowing normally due to the closure of the peripheral blood vessels, such as in the hands and feet, continues to increase in patients as they age. After the blood vessels close, it is thought that angiogenesis, the formation of new blood vessels, and functional maturation take place due to the activation of endothelial cells in the blood vessels, but there are actually many patients whose vascular endothelial functions have already declined due to age or lifestyle-related disease, so sufficient results cannot be expected from therapeutic methods derived from these cells. The researchers observed a significant upregulation in TRPC6 mRNA expression in ischemic limbs (restricted blood flow) after creating a hindlimb ischemic model in which the artery in a mouse's hind leg was ligated (a technique in which medical equipment is used to tie and secure part of the body during surgical treatment). When compared to the wild-type mouse, blood-flow recovery was promoted in the TRPC6 knock-out mouse, and so it became clear that TRPC6 hinders post-ischemic blood-flow recovery.
Moreover, when TRPC6 proteins were phosphorylated in wild-type mice after they were subjected to ischemia, blood-flow recovery was promoted, and the researchers saw an increase in the number of CD31 positive capillaries (an indicator of new blood vessels) and an increased blood vessel diameter, visible when vascular smooth muscle cells underwent muscle differentiation. From this, they learned that the inhibition of channel activity through the phosphorylation of TRPC6 encourages angiogenesis and vascular maturation.
They also verified the effectiveness of blood-flow recovery in relation to ischemia using transgenic mice with overexpression of vascular smooth muscle specific TRPC6 (wild-type) and TRPC6 phosphorylation mutant (T69A: phosphorylation-dead mutant) in TRPC6 knockout mice. Their results showed that while blood-flow recovery was visible in the mice with TRPC6 (wild-type) expression due to TRPC6 phosphorylation, it was not seen in the mice with TRPC6 phosphorylation-dead mutant (T69A) expression.
It became clear that in mice, blood-flow recovery is encouraged by the suppression of TRPC6 channel activity, without dependence on vascular endothelial functions, as blood flow recovered in a similar way in wild-type mice and in ApoE knockout mice (an atherosclerosis model that has inhibited vascular endothelial functions) subjected to ischemia and given TRPC6 inhibitors. Through this, the group identified 1-BP, which promotes blood-flow recovery the most among TRPC6 inhibitors.
Nishida commented, "TRP channels are sensors that are vital to cells, and are famous as the topic of the 2021 Nobel Prize in Physiology or Medicine. We have had many hints about their relationship with disease, but as yet we haven't developed drugs that target them. We aim to develop groundbreaking drugs (TRP channel inhibitors) based on our research."
Journal Information
Publication: British journal of pharmacology
Title: Inhibition of TRPC6 promotes capillary arterialization during post-ischemic blood flow recovery
DOI: 10.1111/bph.15942
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




