When a concentrated solution of salt (NaCl) is stirred, fine crystals appear immediately. However, large crystals grow slowly in the solution if it is not stimulated. Most people know of this phenomenon because of elementary school science experiments, but the formation of tiny clusters of NaCl on the surface of crystals and their smooth diffusion across the surface is difficult to interpret using conventional crystal growth theory. University Professor Eiichi Nakamura of the Department of Chemistry, School of Science, the University of Tokyo, and his research group have successfully visualized the acceleration of the growth of salt crystals by mechanical stimulation using high-speed, high-resolution single-molecule atomic-resolution time-resolved electron microscopy (SMART-EM) that can see individual atoms on a millisecond time scale.
Copyright: @2022 American Chemical Society
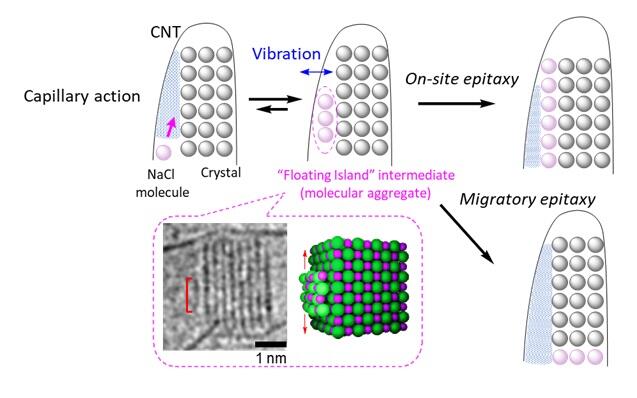
Last year, the research group used electron microscopy to capture every single aspect of the moment of crystallization (colloquially, the birth of baby crystals). Now, they were able to record high-speed images of these baby crystals as they grew in their slowly vibrating crystalline cradle. What they found was that "floating island" intermediates, which have aperiodic structures, and move about quickly, are formed in the preliminary stage of incorporating molecules (ion pairs) into crystals. These intermediates have been overlooked in conventional crystal growth theory.
In addition, the researchers found that crystals grow by capillary action in the nanospace created between the inner wall of the container used to grow crystals and the crystal surface, sucking in floating islands, and that the surface energy of the nanospace changes with each oscillation of the crystal in the container, resulting in the continuous stabilization and destabilization of the floating islands.
"We have quantitatively demonstrated that this floating intermediate vibrates when subjected to an external mechanical stimulus and contributes to increasing the crystal growth rate," explained Nakamura. "This corresponds to the phenomenon observed in everyday life and in scientific experiments, where crystallization is accelerated by a mechanical stimulus, such as stirring a solution. Mechanical stimuli have been overlooked in scientific phenomena. However, their use is expected to allow precise control not only of crystallization, but also of a wide range of chemical phenomena."
Journal Information
Publication: ACS Central Science
Title: Cinematographic Recording of a Metastable Floating Island in Two- and Three-Dimensional Crystal Growth
DOI: 10.1021/acscentsci.2c01093
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




