Research and development aiming to use mRNA (messenger RNA) in pharmaceuticals other than vaccines is progressing around the world, but the instability of mRNA in cells is a major issue. A research group consisting of Graduate Student Shigetoshi Kameda and Professor Hirohide Saito of the Center for iPS Cell Research and Application, Kyoto University (CiRA)and their colleagues has developed two types of circular RNA switches, which make it possible to control genetic expression based on cell type by detecting microRNA (miRNA) and RNA bonding proteins. They have also demonstrated that stable and persistent gene delivery is possible via synthetic circular RNA that contains these switches. ′By combining switch and circular RNA technology, we want to realize mRNA medicine that selectively removes or regenerates only target cells ′ commented Saito. Their research was published in Nucleic Acids Research.

Provided by CiRA, Kyoto University
Gene delivery technologies using synthetic mRNA have highly efficient delivery with a low risk of genome damage and, theoretically, it is possible to add the ability to synthesize all kinds of proteins. These technologies can therefore be applied to areas such as gene therapies and genome editing treatments, not just in vaccines. However, mRNA is unstable in cells, so the persistence of gene expression is low, and gene expression outside of target cells and tissues is also an issue.
The research group focused on circular RNA. Kameda explained, ′In research on gene replacement therapies using mice, the mRNA degrades and has to be administered multiple times, and there are also significant side effects. My initial motivation was that I wanted to avoid these.′
Normal RNA degrades from the ends, but the ends of the circular RNA are closed, so it is unlikely to degrade inside the body and has a good chance for persistent gene expression. However, the technology required to control protein expression from circular RNA did not exist.
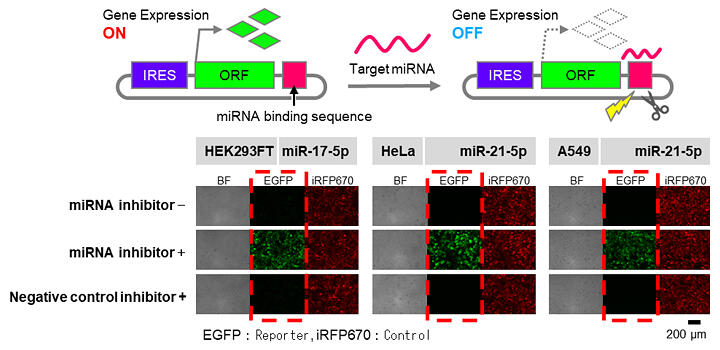
In the body, miRNA represses gene expression by cleaving mRNA with complementary sequences, and RNA bonding proteins (RBP) control functions by bonding to transcribed RNA. These both differ depending on the type of cell and interact with circular RNA and mRNA. The group designed circular RNA, believing that they could control gene expression by imitating and altering the mechanism of this interaction.
Specifically, they first synthesized RNA with an internal ribosome entry site (IRES; this enables the induction of translation in a cap-independent manner) upstream of the gene-coding region, making it into a circular shape. Next, they synthesized circular RNA that controls genetic expression in response to miRNA in cells, succeeding in repressing gene expression only when specific miRNA was present in the cell. They also managed to repress gene expression through RBP - they accomplished this by inserting sequences that sense RBP into IRES sequences needed for circular RNA gene expression. In other words, they created two off switches. Furthermore, they succeeded in creating synthetic gene circuits that combine the two circular RNA switches and activating gene expression in the presence of miRNA.
The output of the 'on' circuit increases depending on the quantity of the target miRNA, and this has better expression persistence than regular mRNA circuits - in this experiment, the group was able to confirm up to 92 times the persistence.
Using this system makes it possible to turn gene expression off in cells containing only the target miRNA, on when the target miRNA and RBP are not present, and off in cells containing only the target RBP. If this could be applied to medicine, it could enable actions such as transforming just mutated cells into normal cells by delivering genes to specific cells only, and removing only the harmful cells that cause disease, such as cancer cells, from among the various cells.
Provided by CiRA, Kyoto University
Saito commented, ′In this research, we were able to develop technology that will become the foundation for new applications of mRNA in medicine. There are high expectations for circular RNA across the globe, and the competition in R&D is intensifying - we are also starting a bio-venture company. We are furthering our research and development in collaboration with various companies and universities, including drug delivery systems, one issue for practical implementation.′
Journal Information
Publication: Nucleic Acids Research
Title: Synthetic circular RNA switches and circuits that control protein expression in mammalian cells
DOI: 10.1093/nar/gkac1252
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




