What is required for the practical application of AI in medicine? This series of articles has explored examples showing the conviction of doctors who want to do something about the problems in medical settings. To accomplish this, they set challenges that current AI technology might be able to do something about and engage in AI development. They try using actual medical information, and feed-back their outcomes into AI technological development, thus carrying out the development of AI that can be used in medical settings. Why was this ideal ecosystem able to form? Professor Toshio Miyata from Tohoku University's Center for Medicinal-Hub says it's to do with 'open innovation, cooperative industry across different businesses and building up experience.'

Provided by Tohoku University
In the past, medicine was centered on pharmaceuticals made up of low-molecular-weight compounds, but with the appearance of biologics such as antibodies and proteins - so-called biopharmaceuticals - this has changed a great deal. New modalities have appeared since the beginning of the 21st century, including gene therapy, cell therapy and nucleic acid medicine, leading to a shift from blockbuster drugs targeting large numbers of patients to individualized medical treatments. Meanwhile, medicine has entered a new phase due to its expansion into biochemistry and molecular biology from pathology and physiology, and the incorporation of medical engineering and computer science.
'The importance of the role of academia as a leader of medical innovation is increasing. In addition to chemistry and biology, science and technology related to engineering and information (and combinations thereof) are considered necessary, and interdisciplinary open innovation is becoming increasingly vital. Moreover, as in the past, it is difficult for a single pharmaceutical company to develop everything, so cooperative industry with different businesses such as venture and IT companies is essential. In the case of university research, I also believe it is difficult for public funding to cover all translational research, including investigator-initiated clinical trials. The way investment funding, including venture capital, is used to advance research and development is significant. Plus, a single university is limited in what it can accomplish, but many universities in the same country working together can manifest great power. Some investigator initiated clinical trials were made possible through the collaboration of 20 or more university medical institutions,' Miyata explained.
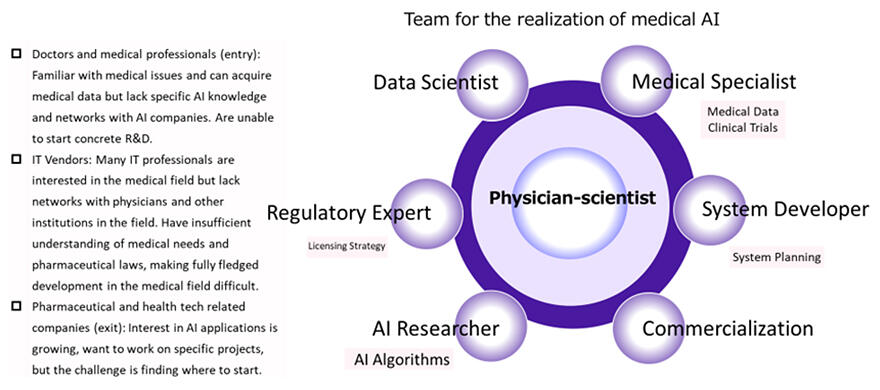
In fact, the Medicinal-Hub develops pharmaceuticals such as PAI-1 inhibitors, medical equipment such as an ultrafine endoscope, and the Software as a Medical Device (artificial intelligence, AI), introduced in this article, through open innovation. Notably, AI development differs from pharmaceuticals and medical equipment, with cross-industrial collaboration as the vital key. 'The use of AI in medicine is a theme that has great potential, but the people involved in planning, who have an important role in research and development, each have their own problems. Although doctors and medical institutions are familiar with medical issues and challenges (needs) and have an abundance of medical data and ideas, they have little knowledge of AI and minimal networks with AI vendors, so they can't launch concrete research and development.
Meanwhile, IT vendors who possess AI technology are interested in applying this to the medical field, but they have few networks with doctors and medical institutions, and they also lack experience with medical needs and pharmaceutical affairs administration such as the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices, making it difficult to carry out full-fledged development in medical settings. On top of this, outlet pharmaceutical and healthtech companies that want to commercialize the application of AI in medicine often find it difficult to handle everything from research to business development themselves from the perspective of time and resources,' said Miyata.
Provided by Tohoku University
To face these challenges, the Tohoku University Center for Medicinal-Hub is focusing on building an ecosystem that connects AI research in the medical field to business, from a perspective that emphasizes understanding medical needs and development in medical settings. It functions as a node that connects doctors, medical institutions, IT vendors with AI technology and outlet pharmaceutical and venture companies, based on experience and knowledge built up through networking with numerous doctors and hospital departments and investigator initiated clinical trials of pharmaceuticals and medical equipment. The cases introduced previously are part of their achievements. When it comes to research and development funding, the Hub uses funds from participating companies such as the bio-venture company Renascience, as well as public funds from AMED and similar organizations.
Miyata commented, 'Over the last 10 or so years, we have engaged in 22 investigator-initiated clinical trials of unapproved medicines, and we are using that experience in a variety of projects today. We are carrying out around seven AI development projects, but in the next few years we hope to increase that to around 20. By putting AI into practice in various medical fields and forms of medical information (figures, audio, images, text), we can see the challenges of medical AI development, and gain an understanding of how we can succeed with medical AI as lots of people involved build up experience. Carrying out repeated implementation together is vital.'
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




