A research group consisting of Assistant Professor Yuta Shirogane of the Faculty of Medical Sciences, Kyushu University, Hidetaka Harada, a student at the School of Medicine, Kyushu University and their colleagues has announced that, working with the National Research Center for the Control and Prevention of Infectious Diseases, Nagasaki University and the Institute for Life and Medical Sciences, Kyoto University, they have clarified the mechanism by which the measles virus causes inflammation in the brain, despite the normal form not having the ability to infect neurons. They analyzed the membrane fusion (F) protein genes of the measles virus derived from patients with inflammation in the brain. The group learned that interactions between mutant F proteins and regular proteins determines how the virus proliferates. It is thought that this is common among viruses that use membrane fusion proteins to cause infection, and it is hoped that this will lead to the development of treatments. The group's results were published in the January 27 edition of the international science journal Science Advances.
Kyushu University/Hidetaka Harada/Yuta Shirogane
The measles virus is the cause of measles, a highly contagious virus. The WHO estimates that in FY2021, 9 million people contracted measles worldwide, and 128,000 people died from it. Measles can be prevented with a vaccination, and is not indigenous to Japan, but infections occur sporadically in the country due to influxes from overseas. In developed countries, the mortality rate is around 0.1%, but in developing countries it is 5%.
In rare cases, the measles virus persistently infects the brain, causing subacute sclerosing panencephalitis (SSPE), in which neurological symptoms progress following a period of viral incubation that lasts several months to several years. Most of these cases occur in infants under five years of age, and progression can be delayed with treatment, but there is no cure.
The measles virus is enveloped by a lipid bilayer, on which receptor binding (H) proteins and F proteins exist and work in concert. In regular infection, the H receptor of the virus particle binds to the target cell receptor and the F protein alters its structure, infecting the target cell by fusing the cell membrane and the envelope.
Previously, the research group has clarified that the virus normally infects immune system cells or epithelial cells, but if the F gene acquires multiple specific mutations, it becomes possible for the measles virus, which does not normally have the ability to propagate in the brain, to propagate in neurons. These mutations enable the virus to express H and F proteins on the surface of infected neurons and spread the infection through membrane fusion with other neuron cells via the synapses.
In this study, the research group carried out genetic analysis of measles viruses isolated from SSPE patients. When they did so, they found that the virus persistently infecting the brain had accumulated more diverse mutations than assumed in its F gene. The group investigated changes in function caused by F gene mutations using a membrane fusion assay system and discovered that while fusogenicity increased due to the accumulated mutations in the F gene in certain patients, it actually decreased in others. Normal progression moves in the direction of high fusogenicity, and the group were not able to explain this decrease.
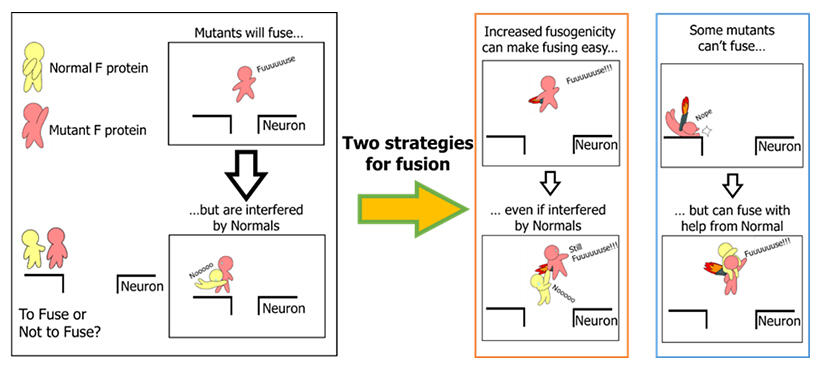
Thus, they focused on the fact that infection transmission in neurons spreads via cell‐cell membrane fusion, causing 'en bloc transmission', in which multiple viral genomes are transmitted simultaneously. In these cases, the mutant F proteins and the regular F proteins co‐exist.
To replicate this, the group coexpressed regular F proteins and mutant F proteins and evaluated the fusogenicity.
F proteins form a trimer (a three‐molecule shape), and the group's outcomes showed that mutant F proteins form a heterotrimer with regular F proteins, which suppresses fusogenicity, but further accumulation of mutations overcomes this suppression. Moreover, the mutant F protein in which fusogenicity is reduced actually increases in fusogenicity in the presence of regular F proteins. This indicates that the virus behaves like a collective, and multiple genomes interact within groups.
Shirogane commented, 'We are still at the basic research stage, but in the future, I hope this will lead to the development of new types of treatment drug that will target viral interactions. I also want to clarify the common mechanism of progression among envelope viruses, including COVID‐19 and the herpes virus.'
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




