A research group led by Tomo‐o Terasawa, a Researcher in the Research Group for Surface and Interface Science of the Advanced Science Research Center at the Japan Atomic Energy Agency, Associate Professor Takahiro Ito of the Synchrotron Radiation Research Center at Nagoya University and Associate Professor Shin‐ichiro Tanaka of the Institute of Scientific and Industrial Research at Osaka University, announced that they have identified the mechanism of the formation of the chemical bond between graphene and gold. The researchers found that the periodicity of the corrugated gold structure and the orientation in which both atoms are aligned are important for the chemical bond between graphene and gold by examining the electron orbitals using angle‐resolved photoemission spectroscopy. Their results are expected to lead to the development of next‐generation energy‐saving integrated circuits and other applications using spin. The group's research was published in the 11 January issue of Physical Review Materials.
Provided by JAEA
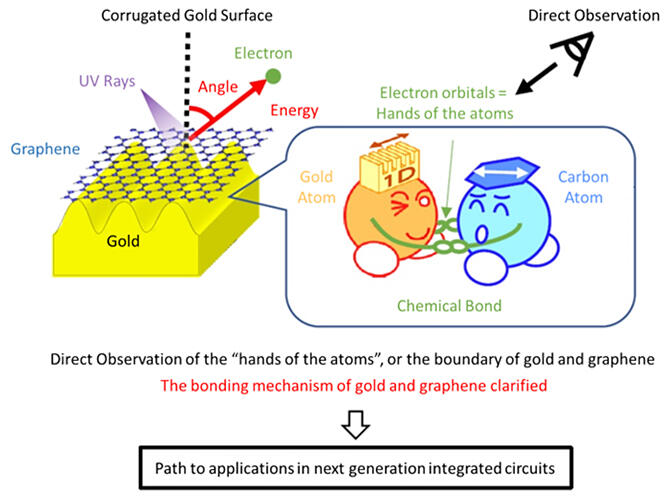
Both graphene and gold are known to be chemically stable and do not easily form chemical bonds with other substances, but they have been reported to form chemical bonds under limited conditions for over a decade. Graphene has no spin bias while gold has a bias on its surface, and chemical coupling could introduce a spin bias from gold to graphene. Therefore, boundaries with chemical bonds between graphene and gold are expected to have applications in spintronic devices. However, it was unclear what arrangement of atoms formed the chemical bonds.
The research group therefore focused on the gold Hex‐Au (001) periodic corrugated structure, which had previously been known to be capable of growing graphene on its surface. The first aim was to determine the electronic structure of the boundary between graphene and gold.
Specifically, they fabricated graphene on the gold surface of the same structure by chemical vapour deposition. They then applied angle‐resolved photoemission spectroscopy, which can reveal the electron orbitals by analyzing the energy and angle of the electrons ejected by UV irradiation. This was compared with the results of graphene fabrication on a flat gold surface.
As a result, gold and graphene electron orbitals were observed on gold surfaces with corrugated structures. However, no gold electron orbitals were observed on the flat gold surface and the graphene electron orbitals were observed as a single straight line.
Furthermore, they succeeded for the first time in the world in observing a bandgap, a region where no electron orbitals exist, in a region deeper than the intersection of the electron orbital called the gold 6s in the corrugated structure of gold and the electron orbit in graphene. Bandgaps are also formed when chemical bonds create new electron orbitals.
The corrugated structure thus modifies the properties of the 6s electron orbital of gold, which is coupled to the electron orbital of graphene. The researchers also demonstrated the possibility of manipulating the position of chemical bonds by controlling the termination of the corrugated structure.
Theoretical calculations also reproduced the observations well, confirming the existence of chemical bonds due to the bandgap.
When the orientation of the gold and graphene crystals is parallel, only the 5d electron orbitals of graphene and gold are bonded, and when this orientation is perpendicular, not only the gold 5d electron orbitals but also the 6s electron orbitals are expected to have bonding. The research group will now verify whether spin bias is actually introduced.
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




