Associate Professor Michio Nakaya of the Graduate School of Pharmaceutical Sciences at Kyushu University, Professor Hidetaka Kosako of the Institute of Advanced Medical Sciences at Tokushima University, Professor Akira Tanaka of Jichi Medical University, Professor Masaki Ohmuraya of Hyogo College of Medicine are the first in the world to find that the protein VGLL3 promotes fibrosis in organs such as myocardial infarction and liver cirrhosis. "We would like to investigate the process of fibrosis itself more deeply, and at the same time, would like to move forward with the development of fibrosis drugs," commented Nakaya. "We are thinking of developing nucleic acid drugs that inhibit the expression of VGLL3 and the approach of degrading VGLL3 with ubiquitination." The group's research was published on 8 February in Nature Communications.
Provided by Kyushu University
In diseased organs, fibroblasts differentiate into myofibroblasts, which produce large amounts of extracellular matrix and undergo fibrosis. Originally a function to repair wounds after trauma, once fibrosis starts in a diseased organ, it snowballs and progresses rapidly.
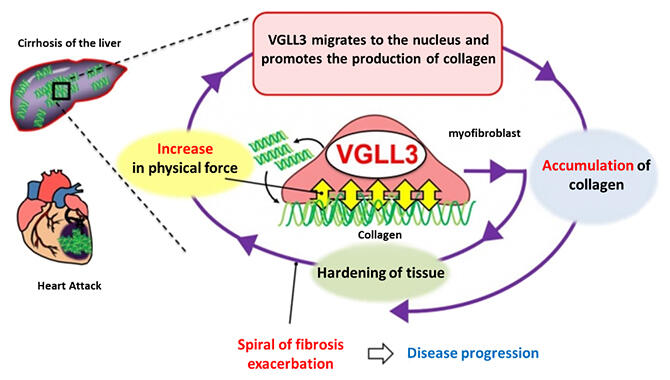
It is known that the key to this accelerated progression of fibrosis is the physical force myofibroblasts receive from the 'hardness' of the collagen fibers that surround them. In other words, when myofibroblasts overproduce collagen around them, collagen fibers accumulate around the cells. Excess collagen fibers become rigid and myofibroblasts are subjected to physical forces from the collagen fibers. When myofibroblasts receive this physical force via their cell surface, intracellular signalling occurs and myofibroblasts go on to produce more extracellular matrix proteins such as collagen. This results in a vicious circle in which the area around the myofibroblasts becomes more rigid, leading to further overproduction of collagen. This is known as negative feedback.
Until now, the mechanism by which myofibroblasts subjected to physical force overproduce collagen in their cells has been poorly understood.
The research group first found that when myofibroblasts from mouse hearts were cultured in plates, the number of myofibroblasts increased when they were cultured attached to the bottom. However, when myofibroblasts were cultured floating in low‐adsorption plates, they dedifferentiated. They also found that when then they returned them to a normal plate and cultured them on the bottom, they re‐differentiated into myofibroblasts. In this study, the researchers examined the differences in gene expression between myofibroblasts and dedifferentiated fibroblasts and found that the transcription‐conjugating factor VGLL3 was expressed only in myofibroblasts that had differentiated after physical stimulation.
Examination of mouse cardiomyocytes showed that VGLL3 was expressed in large amounts in the myocardial infarction model, whereas normal mouse hearts expressed little VGLL3. Further examination of autopsy samples from human myocardial infarction patients showed that VGLL3 was also expressed in fibrotic human hearts.
The researchers placed myofibroblasts from mouse hearts on soft (1 kPa) hydrogels mimicking normal cells and on stiff (50 kPa) hydrogels mimicking fibrotic cells to confirm the intracellular location of VGLL3. They found that VGLL3 migrates from the cytoplasm to the nucleus when subjected to physical stimulation depending on the stiffness. In the nucleus, VGLL3 bound to EWSR1 and promoted collagen production by suppressing the production of miRNA, miR‐29b, which degrades collagen.
They also found that myocardial infarction models in VGLL3 knockout mice showed a reduced degree of fibrosis after myocardial infarction compared to wild‐type models. Furthermore, heart function was also restored after myocardial infarction in the knockout mice.
This study identified VGLL3 as a protein that is important for further collagen production by myofibroblasts under physical force. The discovery of VGLL3 is likely to accelerate the development of drugs for fibrosis, which is responsible for 45% of deaths in developed countries, although the lack of a drug target has prevented the development of drugs for fibrosis.
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




