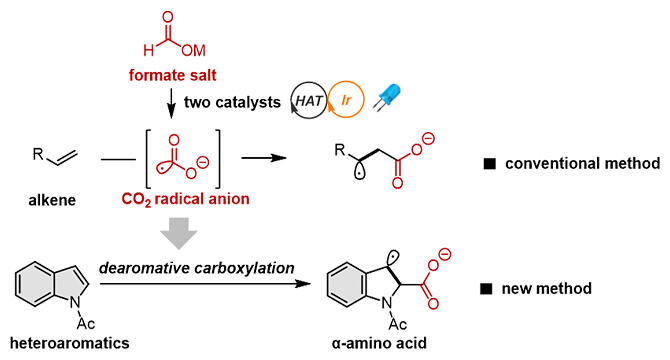
A research group led by Specially Appointed Associate Professor Tsuyoshi Mita at the Institute for Chemical Reaction Design and Discovery (WPI‐ICReDD), Hokkaido University Creative Research Institution, has developed a novel reaction that introduces a carboxyl group into a chemically stable heteroaromatic ring such as indole derivatives using an inexpensive and readily available metal formate (H‐CO2M, where M is an alkali metal) as a single‐carbon resource, and enabled the chemical synthesis of α‐amino acids.
Provided by Hokkaido University
The research group has found that, instead of an electrolytic reaction, irradiation with visible light can similarly generate CO2 radical anions and add them to heteroaromatic rings by using formate and two types of catalyst. By calculating the redox potential of the raw materials and the reaction mechanism using quantum chemical calculations (artificial force induced reaction method, or AFIR method) and other methods, and by efficiently developing the reaction, the carboxylation reaction was successfully developed not only for heteroaromatic rings but also for aromatic rings such as naphthalene. Finally, a carboxylation reaction was found to occur efficiently not only for indoles but also for benzofurans, benzothiophenes and naphthalene derivatives.
Some of the carboxylated forms obtained were themselves α‐amino acids, and chemical transformations that could lead to other compounds with potential as synthetic intermediates for pharmaceuticals. When naphthalene derivatives were used as a raw material, the carboxylation reaction and the reduction of the double bond proceeded simultaneously, an unprecedented reaction format.
CO2 is electrophilic (a substance that seeks electrons) and the radical anion of CO2 is nucleophilic (a substance that gives up electrons) and has different properties. The use of CO2 radical anions may allow stable compounds that would not normally react to be used in the reaction.
"By efficiently using computational chemistry, we want to conduct synthetic chemistry experiments to see what compounds react with other compounds, and develop reaction formats that have not been realized so far," said Mita. "CO2 radical anions can be easily formed by reducing the electrons of CO2 or electron oxidation of formate. The resulting compound is always a carboxylic acid, making it a very simple and useful reaction suitable for the synthesis of medical and agrochemical products."
■ Indole: An aromatic compound consisting of a pyrrole ring fused to a benzene ring. It is also found in many partial frameworks of natural organic compounds and shows aromatic properties.
Journal Information
Publication: ACS Catalysis
Title: Photoredox/HAT‐Catalyzed Dearomative Nucleophilic Addition of the CO2 Radical Anion to (Hetero)Aromatics
DOI: 10.1021/acscatal.2c06192
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




