A research group led by Assistant Professor Yuki Nagashima, graduate student Shiho Ishigaki and Professor Ken Tanaka of the Department of Chemical Science and Engineering of the School of Materials and Chemical Engineering at Tokyo Institute of Technology, and Professor Masanobu Uchiyama of the Graduate School of Pharmaceutical Sciences at The University of Tokyo, has developed a new molecular conversion reaction to synthesize 3D compounds while directly inserting carbon, boron and silicon from 2D compounds.
Provided by Tokyo Institute of Technology
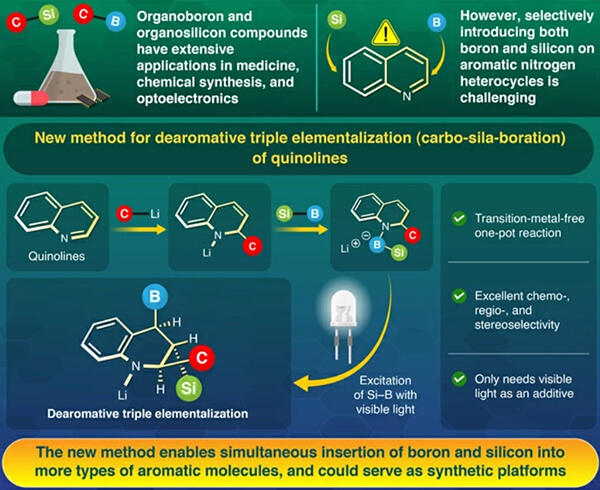
The research group conducted research after realizing that introducing boron and silicon into a two‐dimensional compound to make it three‐dimensional would be an attractive molecular transformation. The addition reaction between the two‐dimensional compound quinoline and an alkyl lithium reagent generates a nitrogen anion (an anion is a negatively charged ionic molecule).
They subsequently examined the reaction conditions under which the dearomative carbon‐boron‐silication reaction proceeds by adding silylborane with a silicon‐boron bond to the nitrogen anion molecule to form a borate complex.
However, irradiation with short wavelength UV light and the use of high temperature conditions resulted in complex mixtures. Due to this, the group conducted a density functional theory computer simulation to activate the silicon‐boron bond under milder conditions, and found that the molecules expected to be the reaction intermediates could absorb visible light in the near blue spectrum.
After irradiation with white or blue light, which has a longer wavelength and is milder, they were able to obtain a high yield of the desired three‐dimensional compound tetrahydroquinoline. The structure of the obtained molecules was determined by single‐crystal X‐ray crystallographic analysis, which confirmed that they selectively obtained compounds in which carbon, silicon and boron were inserted at fixed positions and in opposite stereo configurations to each other. The researchers were able to synthesize 28 3D compound tetrahydroquinolines by applying it to various other quinolines (2D compounds).
The conversion of the boron and silicon functional groups of the synthesized tetrahydroquinolines confirmed that this molecule could serve as a synthetic platform in the search for candidate drug compounds. Furthermore, as raw material two‐dimensional compounds, the method applied not only to quinoline but also to anthracene and phenanthrene, aromatic hydrocarbons widely used in the field of functional materials, and the researchers successfully synthesized the corresponding three‐dimensional compounds. In addition, they analyzed the mechanism of the developed reaction with experimental and computational chemical approaches, which revealed that the silicon‐boron bond is cleaved by light and radicals are generated to promote the reaction.
"It was very difficult to find the right reaction conditions, but in the end, we found that the use of mild visible light energy worked well," said Nagashima. "Moving forward, we would like to use the various three‐dimensional boron‐silicon compounds obtained to develop them into pharmaceuticals, bioactive substances and organic materials."
■ Borate complex: A complex in which an anion is bound to the empty molecular orbital of boron.
■ Quinoline: A nitrogen‐containing 2D planar compound represented by the molecular formula C9H7N.
Journal Information
Publication: Nature Communications
Title: Dearomative triple elementalization of quinolines driven by visible light
DOI: 10.1038/s41467‐023‐36161‐4
This article has been translated by JST with permission from The Science News Ltd. (https://sci-news.co.jp/). Unauthorized reproduction of the article and photographs is prohibited.




